Difference between revisions of "Welcome to the In-Silico Model of butyrolactone regulation in Streptomyces coelicolor"
(→Description of the Model) |
(→Description of the Model) |
||
| Line 22: | Line 22: | ||
Image:Flowchart.png|center|600px | Image:Flowchart.png|center|600px | ||
| − | rect 315 62 327 76 [[Binding of R2 to OR operator |Binding of | + | rect 315 62 327 76 [[Binding of R2 to OR operator |Binding of R2 to OR operator]] |
| − | rect 351 148 363 161 [[Binding of R2 to OA operator |Binding of | + | rect 351 148 363 161 [[Binding of R2 to OA operator |Binding of R2 to OA operator]] |
| − | rect 382 158 397 180 [[Binding of A-R2 to OA' operator |Binding of A- | + | rect 382 158 397 180 [[Binding of A-R2 to OA' operator |Binding of A-R2 to OA' operator]] |
rect 232 152 243 209 [[Transcription of r|Transcription of r]] | rect 232 152 243 209 [[Transcription of r|Transcription of r]] | ||
rect 473 161 485 211 [[Transcription of a|Transcription of a]] | rect 473 161 485 211 [[Transcription of a|Transcription of a]] | ||
| − | rect 315 221 379 253 [[Antisense interaction between r and a|Antisense interaction between r and | + | rect 315 221 379 253 [[Antisense interaction between r and a|Antisense interaction between r and a]] |
rect 71 217 108 232 [[Degradation of r|Degradation of r]] | rect 71 217 108 232 [[Degradation of r|Degradation of r]] | ||
rect 573 219 612 232 [[Degradation of a|Degradation of a]] | rect 573 219 612 232 [[Degradation of a|Degradation of a]] | ||
| Line 33: | Line 33: | ||
rect 231 240 243 310 [[Translation of R|Translation of R]] | rect 231 240 243 310 [[Translation of R|Translation of R]] | ||
rect 473 243 485 392 [[Translation of A|Translation of A]] | rect 473 243 485 392 [[Translation of A|Translation of A]] | ||
| − | rect 232 351 243 399 [[Formation of homo-dimer R2|Formation of homo-dimer | + | rect 232 351 243 399 [[Formation of homo-dimer R2|Formation of homo-dimer R2]] |
| − | rect 283 416 450 449 [[Binding of R2 to A|Binding of | + | rect 283 416 450 449 [[Binding of R2 to A|Binding of R2 to A]] |
rect 151 317 187 332 [[Degradation of R|Degradation of R]] | rect 151 317 187 332 [[Degradation of R|Degradation of R]] | ||
| − | rect 159 418 197 432 [[Degradation of R2|Degradation of | + | rect 159 418 197 432 [[Degradation of R2|Degradation of R2]] |
rect 505 413 543 427 [[Degradation of A|Degradation of A]] | rect 505 413 543 427 [[Degradation of A|Degradation of A]] | ||
| − | rect 195 453 242 475 [[Binding of R2 to C|Binding of | + | rect 195 453 242 475 [[Binding of R2 to C|Binding of R2 to C]] |
rect 475 449 487 506 [[Synthesis of C|Synthesis of C]] | rect 475 449 487 506 [[Synthesis of C|Synthesis of C]] | ||
| − | rect 68 455 105 471 [[Degradation of C2-R2|Degradation of | + | rect 68 455 105 471 [[Degradation of C2-R2|Degradation of C2-R2]] |
| − | rect 356 517 367 545 [[Degradation of A-R2|Degradation of A- | + | rect 356 517 367 545 [[Degradation of A-R2|Degradation of A-R2]] |
rect 622 408 634 435 [[Degradation of C|Degradation of C]] | rect 622 408 634 435 [[Degradation of C|Degradation of C]] | ||
| − | rect 751 397 762 427 [[Degradation of Ce|Degradation of | + | rect 751 397 762 427 [[Degradation of Ce|Degradation of Ce]] |
| − | rect 657 367 719 391 [[Diffusion of C and Ce|Diffusion of C and | + | rect 657 367 719 391 [[Diffusion of C and Ce|Diffusion of C and Ce]] |
</imagemap> | </imagemap> | ||
Revision as of 01:29, 18 October 2015
Contents
About the Project
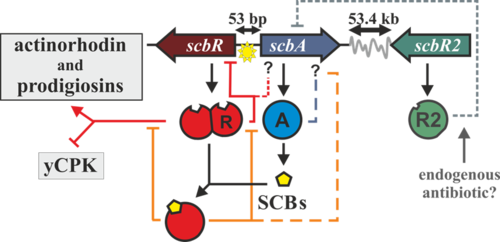
Streptomyces are Gram-positive soil-dwelling bacteria, which are known for being prolific sources of secondary metabolites, many of which have medical interest (e.g.: streptomycin, cloramphenicol or kanamycin). Recent genome sequencing, and posterior bioinformatic analysis, using tools such as antiSMASH [[1]], have shown the presence of putative cryptic secondary metabolite-producing clusters. One way Streptomyces can coordinate secondary metabolite production is through the use of small diffusible molecules, known as γ-butyrolactones. In the model organism Streptomyces coelicolor the butyrolactone regulatory system involves a synthase (scbA) and a butyrolactone receptor (scbR).
The scbA gene is presumed to catalyse the condensation of dihydroxyacetone phosphate with a beta-ketoacid to produce three different butyrolactones (SCB1, SCB2 and SCB3. Hereafter named as SCBs). On the other hand, scbR is a TetR-like DNA-binding protein known to regulate its own transcription and that of scbA and to directly regulate production of a cryptic metabolite and indirectly regulate production of blue pigmented actinorhodin (Act) and prodigiosins (e.g. undecylprodigiosin, Red). These genes are divergently encoded and their promoter regions overlap 53bp.
Transcription analysis have shown that both genes are mainly active during transition from logaritmic growth to stationary phase. It is presumed that SCBs slowly accumulate into the media and upon reaching a concentration threshold promote a coordinated switch-like transition to antibiotic production by binding to ScbR. However, the mechanism of this network is not fully defined, although several alternative scenarios have been proposed. In 2008, Mehra et al [1] proposed a deterministic model involving a putative ScbR-ScbA complex. More recently, Chatterjee et al [2] proposed that the promoter overlap between scbA and scbR is the sole driving force of the precise switch-like transition.
The aim of this project is the design and analysis of a stochastic and parameter uncertainty-aware model which describes the butyrolactone regulatory system and allows reliable predictions of its behaviour. The complete elucidation of this system could potentially lead to the design of robust and sensitive systems as orthologous regulatory circuits in synthetic biology and biotechnology.
Description of the Model
Several alternative scenarios for the mechanism of action of the GBL system have been previously proposed. Our aim is to create a unified model which will include them all and enable their parallel or combined analysis. The scenarios investigated are the following:
- The formation of a complex between ScbA and ScbR proteins (ScbA-ScbR), which relieves ScbR repression, while at the same time activates the transcription of scbA, and in turn of SCBs. [1]
- The effect of transcriptional interference (collisions between the elongating RNAPs which leads to transcriptional termination) due to the overlap of the two genes' promoter regions by 53 bp and by the convergent transcription of the two genes. This results in a decrease in expression of full-length mRNAs from both promoters and production of truncated mRNAs. [2] [3]
- The antisense effect conferred by convergent transcription of the scbR and scbA genes. In this case, transcripts with a segment of complementary sequence may lead to interactions between sense-antisense full length transcripts of the two genes, thus leading to the formation of a fast degrading complex of the two mRNAs and inhibition of translation. [2]
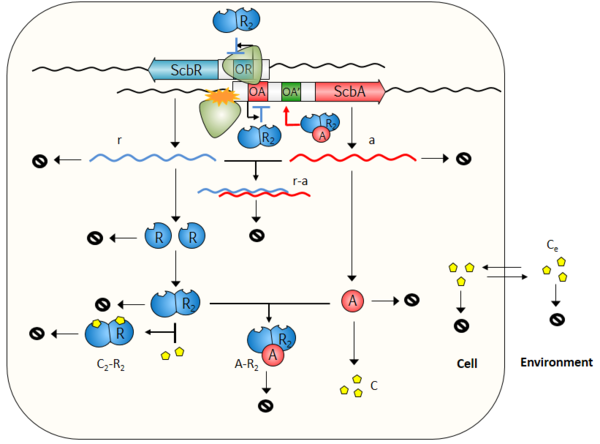
The model consists of two compartments, the Cell and the Environment. A schematic description of the model is provided below. More information can be obtained by clicking on each reaction arrow in the figure.
Species
| Name | Description | Compartment |
|---|---|---|
| OR | Operator site of ScbR upstream scbR promoter | Cell |
| OA | Operator site of ScbR within scbA promoter | Cell |
| OA' | Putative operator site of AR2 complex | Cell |
| OR-R2 | Complex of R protein and operator site upstream scbR promoter | Cell |
| OA-R2 | Complex of R protein and operator site within scbA promoter | Cell |
| OA'-AR2 | Putative operator site presumed to activate scbA gene by the AR2 complex | Cell |
| r | mRNA transcript of scbR gene | Cell |
| a | mRNA transcript of scbA gene | Cell |
| r-a | complex of full length scbR and scbA mRNAs due to antisense effect | Cell |
| R | ScbR protein | Cell |
| R2 | ScbR homo-dimer | Cell |
| A | ScbA protein | Cell |
| C | SCBs (γ-butyrolactones) | Cell |
| AR2 | ScbA-ScbR complex | Cell |
| S | Glycerol derivative and b-keto acid derivative precursors | Cell |
| C2R2 | SCBs-ScbR complex | Cell |
| Ce | Extracellular SCBs (γ-butyrolactones) | Environment |
Reactions
The reactions per compartment are the following:
|
Cell |
Cell-Environment
Environment |
Parameter Overview
In order to create the appropriate probability distributions for the models parameters, reported parameter values in literature and the expertise and knowledge of experimental microbiologists are employed. Hence, we decide on a most plausible value for each parameter, which is the mode (global maximum) of the corresponding distribution (Probability Density Function or PDF), and on a confidence interval, which represents the margin within which is the 95% of the values, in a multiplicative pattern. In this context, if the mode for a parameter value is X, the confidence interval multiplicative factor is y and the range of plausible values is ![[\frac{X}{y},X\cdot y]](/wiki/images/math/e/d/2/ed2bf0bf688aea7be4016cbe08252a9d.png) . From these values, a two-by-two system of the cumulative distribution function (CDF) and the mode equations is solved in order to derive the location parameter Failed to parse (PNG conversion failed; check for correct installation of latex and dvipng (or dvips + gs + convert)): μ
and the scale parameter Failed to parse (PNG conversion failed; check for correct installation of latex and dvipng (or dvips + gs + convert)): σ
of the log-normal distribution. The system is the following:
. From these values, a two-by-two system of the cumulative distribution function (CDF) and the mode equations is solved in order to derive the location parameter Failed to parse (PNG conversion failed; check for correct installation of latex and dvipng (or dvips + gs + convert)): μ
and the scale parameter Failed to parse (PNG conversion failed; check for correct installation of latex and dvipng (or dvips + gs + convert)): σ
of the log-normal distribution. The system is the following:
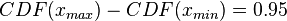
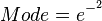
where ![CDF= \frac{1}{2}+\frac{1}{2} \mathrm{erf} \Big[\frac{lnx-μ}{\sqrt{2}σ}\Big]](/wiki/images/math/1/1/b/11bf6de96a49a30625ed407f8c0e31fd.png) and
and 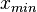 and
and 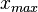 are the minimum and maximum values of the confidence interval.
are the minimum and maximum values of the confidence interval.
An overview for all the parameters of the model can be found here.
References
- ↑ 1.0 1.1 S. Mehra, S. Charaniya, E. Takano, and W.-S. Hu. A bistable gene switch for antibiotic biosynthesis: The butyrolactone regulon in streptomyces coelicolor. PLoS ONE, 3(7), 2008. Cite error: Invalid
<ref>tag; name "Mehra2008" defined multiple times with different content - ↑ 2.0 2.1 2.2 A. Chatterjee, L. Drews, S. Mehra, E. Takano, Y.N. Kaznessis, and W.-S. Hu. Convergent transcription in the butyrolactone regulon in streptomyces coelicolor confers a bistable genetic switch for antibiotic biosynthesis. PLoS ONE, 6(7), 2011.
- ↑ E. Takano. γ-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Current Opinion in Microbiology, 9(3):287–294, 2006.