Degradation of C2-R2
The SCB-ScbR protein complex (C2-R2) degrades.
Contents
Chemical equation
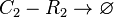
Rate equation
![r= d_{CR}\cdot[C_{2}-R_{2}]](/wiki/images/math/0/0/b/00b0af37b185dcb7094f942404631e79.png)
Parameters
The parameter of this reaction is the degradation rate of C2-R2 (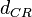 ). The parameter values were derived from proteomics studies on different proteins of S. coelicolor.
). The parameter values were derived from proteomics studies on different proteins of S. coelicolor.
| Name | Value | Units | Value in previous GBL models [1] [2] | Remarks-Reference |
|---|---|---|---|---|
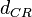
|
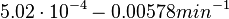 [3] [4] [5] [3] [4] [5]
|

|
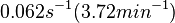 [1] [1]
Range tested: ( Bistability range: ( and ( |
In a quantitative proteomics study on protein turnover rates in dynamic systems, Jayapal et al. reported the degradation rates of 115 proteins in S. coelicolor cultures undergoing transition from exponential growth to stationary phase. The values were in the range 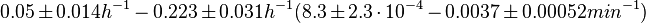 with a median of with a median of 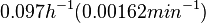 . .
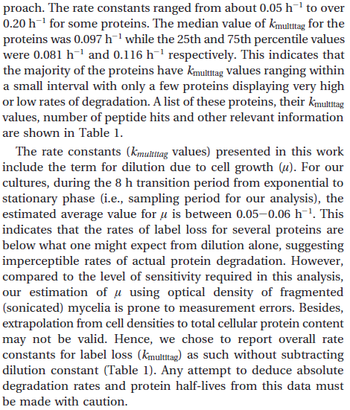 Jayapal et al. 2012[4] The full list of the proteins studied and their turnover rate constants can be found here. Additionally, an in vivo study examining the stability of 184 proteins in growing and non-growing E. coli[5] reported 47 proteins that degraded within 24 hours, while the rest were either modified (27) or were found to be stable (114). The values measured were in the range  Mosteller et al. 2012[5] |
Parameters with uncertainty
When deciding how to describe the uncertainty for this parameter we must take into consideration that the reported values are estimated or calculated from proteomics experiments, using methods which were prone to measurement errors (i.e. optical density of fragmented mycelia to estimate the growth rate (μ)) and extrapolations from cell density to total cellular protein without taking into account the dilution due to cell division. Additionally, the study did not include measurements on protein complexes, although it was conducted on a wide range of S. coelicolor proteins which enables a rough estimation of the range of values of protein degradation in this species. These facts influence the quantification of the parameter uncertainty and therefore the shape of the corresponding distribution. By assigning the appropriate weights to the parameter values and using the method described here, the appropriate probability distribution was designed.
The mode of the log-normal distribution of the 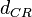 to
to 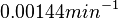 . In order to explore the full range of published values and even sample a percentage of values outside the reported range, the Spread is set to
. In order to explore the full range of published values and even sample a percentage of values outside the reported range, the Spread is set to 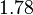 , so that the range where 68.27% of the values are found is between
, so that the range where 68.27% of the values are found is between 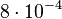 and
and 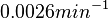 .
.
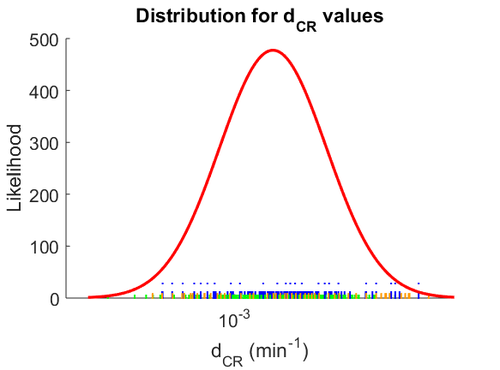
The probability distribution for the parameter, adjusted accordingly in order to reflect the above values, is the following:
The values retrieved from literature and their weights are indicated by the blue dashed lines, and the uncertainty for each value is indicated using the reported experimental error (green lines) or a default value of 10% error (orange lines).
The parameter information of the distribution is:
| Parameter | Mode | Spread | μ | σ |
|---|---|---|---|---|
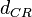
|
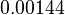
|
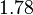
|
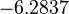
|
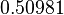
|
References
- ↑ 1.0 1.1 1.2 S. Mehra, S. Charaniya, E. Takano, and W.-S. Hu. A bistable gene switch for antibiotic biosynthesis: The butyrolactone regulon in streptomyces coelicolor. PLoS ONE, 3(7), 2008.
- ↑ 2.0 2.1 2.2 A. Chatterjee, L. Drews, S. Mehra, E. Takano, Y.N. Kaznessis, and W.-S. Hu. Convergent transcription in the butyrolactone regulon in streptomyces coelicolor confers a bistable genetic switch for antibiotic biosynthesis. PLoS ONE, 6(7), 2011.
- ↑ Trötschel C, Albaum SP, Poetsch A. Proteome turnover in bacteria: current status for Corynebacterium glutamicum and related bacteria. Microbial biotechnology. 2013;6(6):708-719.
- ↑ 4.0 4.1 Jayapal KP, Sui S, Philp RJ, Kok YJ, Yap MG, Griffin TJ, Hu WS. Multitagging proteomic strategy to estimate protein turnover rates in dynamic systems. J Proteome Res. 2010 May 7;9(5):2087-97.
- ↑ 5.0 5.1 5.2 Mosteller RD, Goldstein RV, Nishimoto KR. Metabolism of individual proteins in exponentially growing Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2524-32


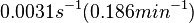
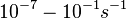
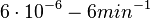 )
)
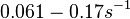
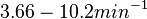 )
)
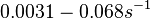
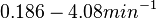 )
)