Difference between revisions of "Extracellular signal-regulated kinase (ERK)"
| (9 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
<td style="vertical-align:top;"> | <td style="vertical-align:top;"> | ||
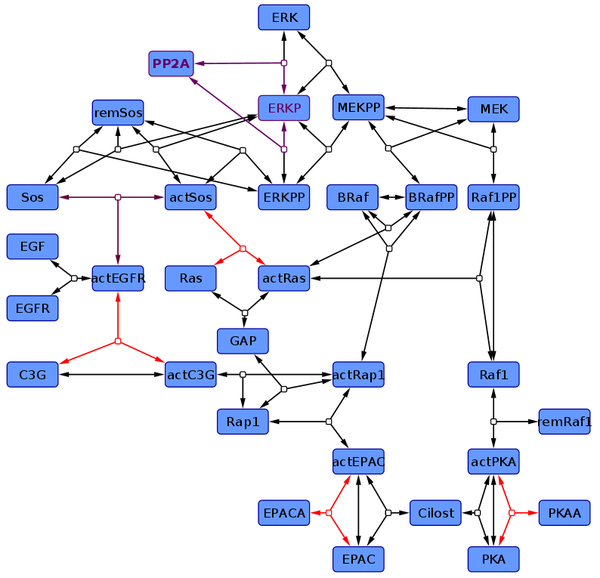
According to the topology which is found as most likely by Xu et al (2010)<ref name="Xu2010"> Tian-Rui Xu et al. (2010) "Inferring signaling pathway topologies from multiple perturbation measurements of specific biochemical species." ''Sci Signal.'' 3(134):ra20. ([http://www.ncbi.nlm.nih.gov/pubmed/20234003 pmid:20234003])</ref> a kinetic model is created. Literature data is used to estimate the parameter values and their uncertainty. <br> | According to the topology which is found as most likely by Xu et al (2010)<ref name="Xu2010"> Tian-Rui Xu et al. (2010) "Inferring signaling pathway topologies from multiple perturbation measurements of specific biochemical species." ''Sci Signal.'' 3(134):ra20. ([http://www.ncbi.nlm.nih.gov/pubmed/20234003 pmid:20234003])</ref> a kinetic model is created. Literature data is used to estimate the parameter values and their uncertainty. <br> | ||
| − | + | This results in a probablitiy distribution for each parameter. According to these distributions samples were drawn and for each parameter composition a steady state analysis was performed. The result of this prcedure is that each output of the model is givn with a certain uncertainty. | |
| + | For more information about previous applications for this kind of uncertainty modelling please refer to the publications listed [[#Previous Publications about uncertainty modelling|here]]. | ||
| − | |||
| − | In one (non-modified model) only one reaction kinetic differs from Xu et al. (2010)<ref name="Xu2010"></ref>, the [[Sos-Binding|Sos-Activation]] (purple reaction) and is assumed be a binding process rather than enzyme catalysed. Furthermore we modelled the [[ERK-Activation]] as two step reaction, with the monophosphorylated ERK as intermediate. | + | Due to the literature data and the parameter measured, we changed the kinetics of some reactions and ended up in two different models: |
| + | |||
| + | In one (non-modified model) only one reaction kinetic differs from Xu et al. (2010)<ref name="Xu2010"></ref>, the [[Sos-Binding|Sos-Activation]] (purple reaction) and is assumed be a binding process rather than enzyme catalysed. Furthermore we modelled the [[ERK-Activation]] as two step reaction, with the monophosphorylated ERK as intermediate. | ||
In the other one (modified model) further kinetics were changed in addition to these modifications (marked in red). So are the [[EPAC_and_PKA-Activation_(Agonist)|activation of EPAC and PKA by an agonist]] modelled as binding, [[C3G-Activation]] is modelled in two steps, first a binding to the receptor than a phosphorylation, and the [[Ras-Activation]] process is splitted in its elementary reactions, and their kinetics are therefore mass action. <br> | In the other one (modified model) further kinetics were changed in addition to these modifications (marked in red). So are the [[EPAC_and_PKA-Activation_(Agonist)|activation of EPAC and PKA by an agonist]] modelled as binding, [[C3G-Activation]] is modelled in two steps, first a binding to the receptor than a phosphorylation, and the [[Ras-Activation]] process is splitted in its elementary reactions, and their kinetics are therefore mass action. <br> | ||
| Line 29: | Line 31: | ||
<imagemap> | <imagemap> | ||
| − | File: | + | File:L_MAPK_komplett.png|frameless|600px |
rect 130 435 179 467 [[C3G-Activation|C3G-Activation]] | rect 130 435 179 467 [[C3G-Activation|C3G-Activation]] | ||
| Line 110: | Line 112: | ||
<h2>Model File </h2> | <h2>Model File </h2> | ||
| + | [[File:MAPK-PPA2.xml]] | ||
<h2>Equilibrium constants</h2> | <h2>Equilibrium constants</h2> | ||
| Line 125: | Line 128: | ||
*Kerkhoven EJ, Achcar F, Alibu VP, Burchmore RJ, Gilbert IH, Trybiło M, Driessen NN, Gilbert D, Breitling R, Bakker BM, Barrett MP. (2013) "Handling uncertainty in dynamic models: the pentose phosphate pathway in Trypanosoma brucei." ''PLoS Comput Biol.'' 9(12):e1003371. [http://www.ncbi.nlm.nih.gov/pubmed/24339766 pmid:24339766] | *Kerkhoven EJ, Achcar F, Alibu VP, Burchmore RJ, Gilbert IH, Trybiło M, Driessen NN, Gilbert D, Breitling R, Bakker BM, Barrett MP. (2013) "Handling uncertainty in dynamic models: the pentose phosphate pathway in Trypanosoma brucei." ''PLoS Comput Biol.'' 9(12):e1003371. [http://www.ncbi.nlm.nih.gov/pubmed/24339766 pmid:24339766] | ||
| + | |||
| + | *[http://2013.igem.org/Team:Manchester/Enzyme Igem Team Manchester 2013] | ||
<h2>References</h2> | <h2>References</h2> | ||
<references/> | <references/> | ||
Latest revision as of 08:15, 3 August 2014
Description of the model
|
According to the topology which is found as most likely by Xu et al (2010)[1] a kinetic model is created. Literature data is used to estimate the parameter values and their uncertainty. For more information about previous applications for this kind of uncertainty modelling please refer to the publications listed here.
Due to the literature data and the parameter measured, we changed the kinetics of some reactions and ended up in two different models: In one (non-modified model) only one reaction kinetic differs from Xu et al. (2010)[1], the Sos-Activation (purple reaction) and is assumed be a binding process rather than enzyme catalysed. Furthermore we modelled the ERK-Activation as two step reaction, with the monophosphorylated ERK as intermediate. In the other one (modified model) further kinetics were changed in addition to these modifications (marked in red). So are the activation of EPAC and PKA by an agonist modelled as binding, C3G-Activation is modelled in two steps, first a binding to the receptor than a phosphorylation, and the Ras-Activation process is splitted in its elementary reactions, and their kinetics are therefore mass action.
|
Reactions
Model File
Equilibrium constants
Parameter Overview
Previous Publications about uncertainty modelling
- Achcar, F., Kerkhoven, E., Bakker, B., Barrett, M. & Breitling, R (2012). "Dynamic modelling under uncertainty: The case of Trypanosoma brucei energy metabolism." PLoS Computational Biology, 8(1):e1002352 pmid:22379410
- Breitling R, Achcar F, Takano E. (2013) "Modeling challenges in the synthetic biology of secondary metabolism." ACS Synth Biol., 2(7):373-8. doi: 10.1021/sb4000228 pmid:23659212
- Achcar F, Barrett MP, Breitling R. (2013) "Explicit consideration of topological and parameter uncertainty gives new insights into a well-established model of glycolysis." FEBS J. 280(18):4640-51 pmid:23865459
- Kerkhoven EJ, Achcar F, Alibu VP, Burchmore RJ, Gilbert IH, Trybiło M, Driessen NN, Gilbert D, Breitling R, Bakker BM, Barrett MP. (2013) "Handling uncertainty in dynamic models: the pentose phosphate pathway in Trypanosoma brucei." PLoS Comput Biol. 9(12):e1003371. pmid:24339766
References
- ↑ 1.0 1.1 1.2 Tian-Rui Xu et al. (2010) "Inferring signaling pathway topologies from multiple perturbation measurements of specific biochemical species." Sci Signal. 3(134):ra20. (pmid:20234003)