Difference between revisions of "Extracellular signal-regulated kinase (ERK)"
m |
m |
||
| Line 9: | Line 9: | ||
<td style="vertical-align:top"> | <td style="vertical-align:top"> | ||
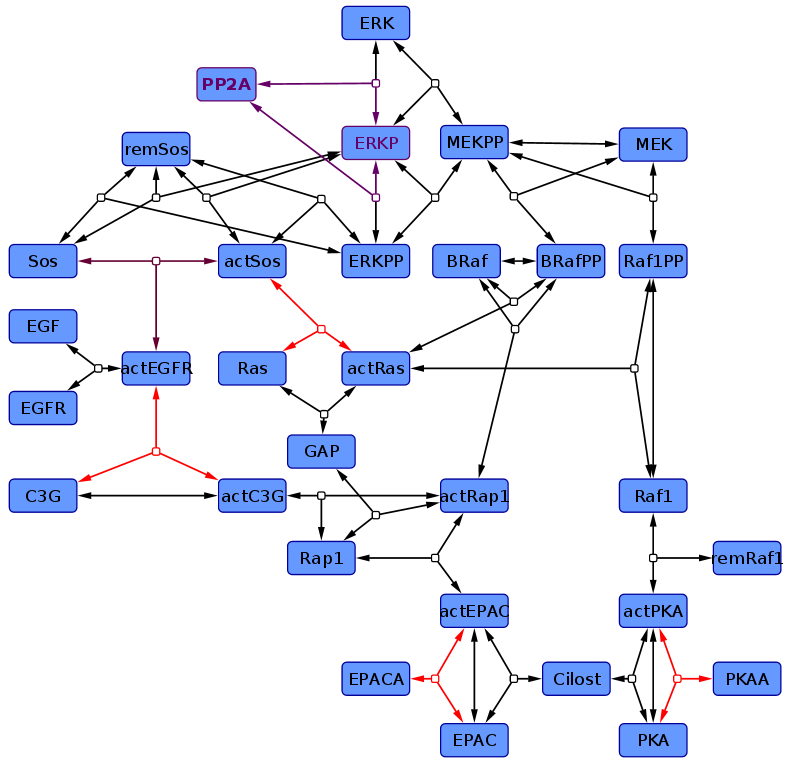
According to the topology which is found as most likely by Xu et al (2010)<ref name="Xu2010"> Tian-Rui Xu et al. (2010) "Inferring signaling pathway topologies from multiple perturbation measurements of specific biochemical species." Sci Signal. 3(134):ra20. ([http://www.ncbi.nlm.nih.gov/pubmed/20234003 pmid:20234003])</ref> a kinetic model is created. Literature data is used to estimate the parameter values and their error. <br> | According to the topology which is found as most likely by Xu et al (2010)<ref name="Xu2010"> Tian-Rui Xu et al. (2010) "Inferring signaling pathway topologies from multiple perturbation measurements of specific biochemical species." Sci Signal. 3(134):ra20. ([http://www.ncbi.nlm.nih.gov/pubmed/20234003 pmid:20234003])</ref> a kinetic model is created. Literature data is used to estimate the parameter values and their error. <br> | ||
| − | We ended up in two different models | + | We ended up in two different models: |
In one (non-modified model) only one reaction kinetic differs from Xu et al. (2010)<ref name="Xu2010"></ref>, the Sos-Activation (purple reaction) and is assumed be a binding process rather than enzyme catalysed. Furthermore we modelled the ERK-Activation as two step reaction, with the monophosphorylated ERK as intermediate. | In one (non-modified model) only one reaction kinetic differs from Xu et al. (2010)<ref name="Xu2010"></ref>, the Sos-Activation (purple reaction) and is assumed be a binding process rather than enzyme catalysed. Furthermore we modelled the ERK-Activation as two step reaction, with the monophosphorylated ERK as intermediate. | ||
Revision as of 17:38, 11 March 2014
Description of the model
|
According to the topology which is found as most likely by Xu et al (2010)[1] a kinetic model is created. Literature data is used to estimate the parameter values and their error. In one (non-modified model) only one reaction kinetic differs from Xu et al. (2010)[1], the Sos-Activation (purple reaction) and is assumed be a binding process rather than enzyme catalysed. Furthermore we modelled the ERK-Activation as two step reaction, with the monophosphorylated ERK as intermediate. In the other one (modified model) further kinetics were changed in addition to these modifications (marked in red). So are the activation of EPAC and PKA by an agonist modelled as binding, C3G-Activation is modelled in two steps, first a binding to the receptor than a phosphorylation, and the Ras-Activation process is splitted in its elementary reactions, and their kinetics are therefore mass action.
|
Reactions
Model File
References
- ↑ 1.0 1.1 1.2 Tian-Rui Xu et al. (2010) "Inferring signaling pathway topologies from multiple perturbation measurements of specific biochemical species." Sci Signal. 3(134):ra20. (pmid:20234003)