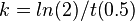Difference between revisions of "Degradation Pathways"
| (24 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
| − | + | To account for the decay in metabolite concentration over time, there are 46 decay reactions in this network. In these reactions, the product mass is pooled together under the term “Miscellaneous metabolites”. This is a collective term which refers to metabolites which are no longer of interest to this work, for example they are exported to the systemic circulation or undergo degradation. To account for the different rate of metabolite decay in the intracellular and extracellular compartments, each metabolite has two decay reactions which are governed by independent parameters. | |
| − | + | To calculate the rate constant, the following equation was used: | |
| − | + | <math> k = ln(2)/t(0.5) </math> | |
| − | + | ||
| − | + | {|width ="80%" | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| − | | | + | * [[Decay of AA to exAA|Decay of AA/exAA (R64/72) ]] |
| − | | | + | * [[Decay of PGI2 to exPGI2 |Decay of PGI<sub>2</sub>/exPGI<sub>2</sub> (R48/77) ]] |
| − | | | + | * [[Decay of PGE2 to exPGE2 |Decay of PGE<sub>2</sub>/exPGE<sub>2</sub> (R49/76) ]] |
| − | | | + | * [[Decay of PGF2a/exPGF2a |Decay of PGF<sub>2a</sub>/exPGF<sub>2a</sub> (R44/74) ]] |
| − | | | + | * [[Decay of PGH2 to exPGH2 |Decay of PGH<sub>2</sub>/exPGH<sub>2</sub> (R53/73) ]] |
| − | + | * [[Decay of PGD2 to exPGD2 |Decay of PGD<sub>2</sub>/exPGD<sub>2</sub> (R52/78) ]] | |
| − | | | + | * [[Decay of PGJ2 to exPGJ2 |Decay of PGJ<sub>2</sub>/exPGJ<sub>2</sub> (R51/83) ]] |
| − | | | + | * [[Decay of TXB2/exTXB2 |Decay of TXB<sub>2</sub>/exTXB<sub>2</sub> (R45/81) ]] |
| − | | | + | * [[Decay of TXA2 to exTXA2 |Decay of TXA<sub>2</sub>/exTXA<sub>2</sub> (R46/75) ]] |
| − | | | + | * [[Decay of K6PGF2a to exK6PGF2a |Decay of 6-keto-PGF<sub>1a</sub>/ex6-keto-PGF<sub>1a</sub> (R47/82)]] |
| − | |- | + | * [[Decay of D15PGJ2 to exD15PGJ2 |Decay of 15-deoxy-PGJ<sub>2</sub>/ex15-deoxy-PGJ<sub>2</sub> (R50/84) ]] |
| − | | | + | * [[Decay of 15-Keto-PGE2 to ex15-Keto-PGE2 |Decay of 15-keto-PGE<sub>2</sub>/ex15-keto-PGE<sub>2</sub> (R68/79)]] |
| − | | | + | * [[Decay of 3,4-Dihydro-15-Keto-PGE2 to ex3,4-Dihydro-15-Keto-PGE2 |Decay of 13,14-dihydro-15-keto-PGE<sub>2</sub>/ex13,14-dihydro-15-keto-PGE<sub>2</sub> (R71/80)]] |
| − | | | + | * [[Decay of 5-Oxo-ETE to ex5-Oxo-ETE |Decay of 5-oxo-ETE/ex5-oxo-ETE (R54/91) ]] |
| − | + | * [[Decay of LTB4 to exLTB4 |Decay of LTB<sub>4</sub>/exLTB<sub>4</sub> (R56/93) ]] | |
| − | + | * [[Decay of LTC4 to exLTC4 |Decay of LTC<sub>4</sub>/exLTC<sub>4</sub> (R57/94) ]] | |
| − | + | * [[Decay of LTA4 to exLTA4 |Decay of LTA<sub>4</sub>/exLTA<sub>4</sub> (R58/92) ]] | |
| − | + | * [[Decay of 12-HPETE to ex12-HPETE |Decay of 12-HPETE/ex12-HPETE (R63/86) ]] | |
| − | + | * [[Decay of 15-HPETE to ex15-HPETE |Decay of 15-HPETE/ex15-HPETE (R61/85)]] | |
| − | + | * [[Decay of 5-HPETE to ex5-HPETE |Decay of 5-HPETE/ex5-HPETE (R59/87) ]] | |
| − | + | * [[Decay of 5-HETE to ex5-HETE |Decay of 5-HETE/ex5-HETE (R55/90) ]] | |
| − | + | * [[Decay of 15-HETE to ex15-HETE |Decay of 15-HETE/ex15-HETE (R60/88) ]] | |
| − | + | * [[Decay of 12-HETE to ex12-HETE |Decay of 12-HETE/ex12-HETE (R62/89) ]] | |
| − | |||
| − | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |- | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |- | ||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|} | |} | ||
| + | |||
| + | |||
| + | |||
| + | == References == | ||
| + | <references/> | ||
Latest revision as of 09:51, 17 May 2019
To account for the decay in metabolite concentration over time, there are 46 decay reactions in this network. In these reactions, the product mass is pooled together under the term “Miscellaneous metabolites”. This is a collective term which refers to metabolites which are no longer of interest to this work, for example they are exported to the systemic circulation or undergo degradation. To account for the different rate of metabolite decay in the intracellular and extracellular compartments, each metabolite has two decay reactions which are governed by independent parameters.
To calculate the rate constant, the following equation was used: