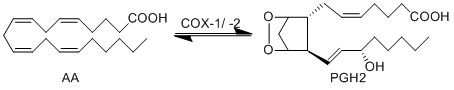
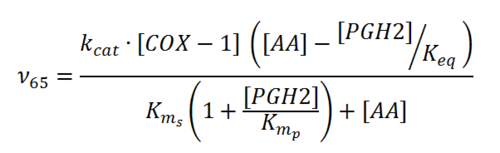Transformation of AA to PGH2 (COX-1)
Cyclooxygenase, COX, also known as prostaglandin endoperoxide H (PGH) synthase, has a broad substrate specificity and is reported to metabolise AA and other fatty acids into prostanoids such as PGs, TXs and prostacyclin [1][2]. The preferred substrate is AA for both isoforms of COX, COX-1 and COX-2. The COX-1 isoform is constitutively expressed, whereas COX-2 expression is typically negligible in normal cells [3] but can be induced in response to inflammatory stimuli, hormones, calcium and growth factors[4][5][6][7][8][9] [10]. Interestingly, basal expression of COX-2 has been found to occur in the kidney, central nervous system, female reproductive organs and stomach [11][12], and frequent expression of COX-2 can be found in the tumorigenic nests of most cancers [13][14].
Both isoforms of COX catalyse a two-step reaction of cycloooxygenation and oxygenation, followed by a hydroperoxide reduction. The cyclooxygenase reaction occurs in the hydrophobic channel within the core of the protein and generates an unstable peroxide intermediate, PGG2. The subsequent peroxidase reaction produces PGH2 and occurs at the heme-containing active site near the protein surface. The two step reaction results in the insertion of molecular oxygen across the C-9 and C-11 double bonds. Both isoforms of COX are heme-containing glycoproteins, that are membrane-bound to the endoplasmic reticulum (ER) and function as homodimers [15][16][17][18]. Interestingly, in certain tissues COX-1 has been found to colocalises preferentially, but not exclusively, with TXAS, PGFS and cPGES, whereas COX-2 has been found to colocalise with PGIS and mPGES in certain tissues [19][20][21][22][23].
Contents
Reaction
Chemical equation
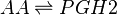
Rate equation
Enzyme Parameters
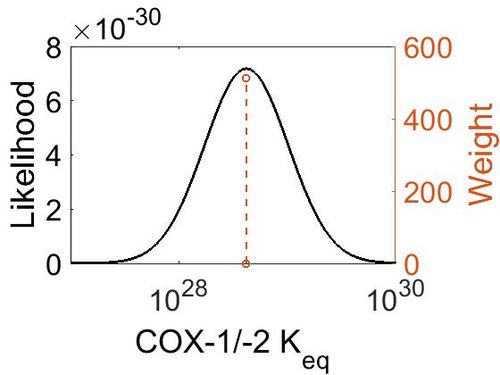
Keq
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| (-30) | kcal/mol | Unspecified | Calculations with a Gaussian98 suite of programs
Enzyme: COX (Unspecific) Substrate: Arachidonate Temperature: 298.15 K Pressure: 1 bar |
64 | [24] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.18E+28 | 1.00E+01 | 6.67E+01 | 8.90E-01 |
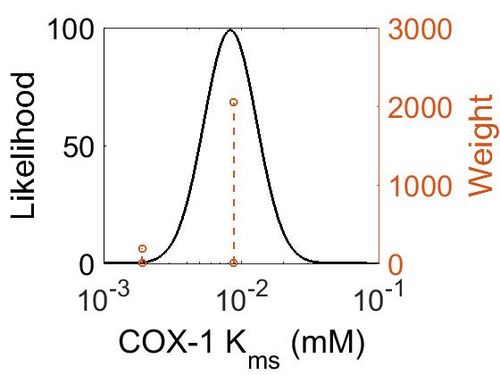
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 0.0088 ± 0.0022 | 
|
Human | Expression Vector: Human
Enzyme: Cyclooxygenase-1 pH: 7.6 - 8.4 Temperature: 37 °C |
2048 | [25] |
| 0.0019 ± 0.0002 | 
|
Ram | Expression Vector: Ram
Enzyme: Cyclooxygenase-1 pH: 8 Temperature: 30 ± 0.2 °C |
192 | [26] |
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 8.30E-03 | 1.62E+00 | -4.60E+00 | 4.41E-01 |
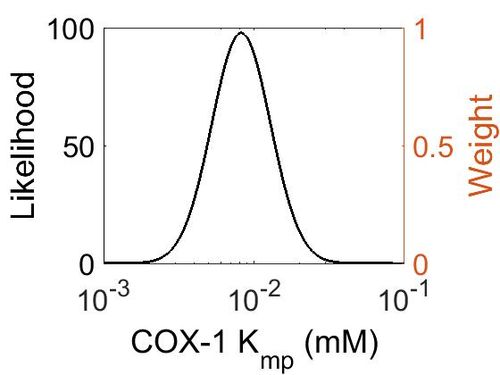
Kmp
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (mM) | Location parameter (μ) | Scale parameter (σ) |
|---|---|---|
| 8.20E-03 | -4.60E+00 | 4.49E-01 |
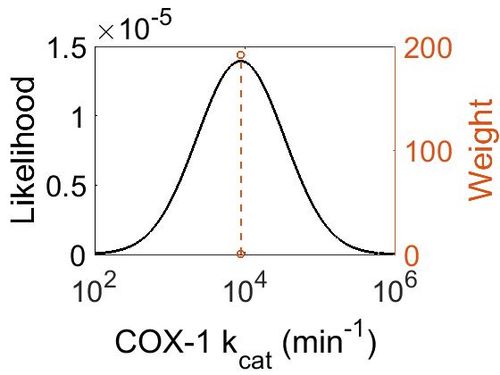
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 8820 ± 360 | 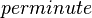
|
Ram | Expression Vector: Ram
Enzyme: Cyclooxygenase-1 pH: 8 Temperature: 30 ± 0.2 °C |
192 | [26] |
| Mode (min-1) | Confidence Interval | Location parameter (μ) | Scale parameter (σ) |
|---|---|---|---|
| 8.81E+03 | 1.04E+00 | 9.09E+00 | 4.08E-02 |
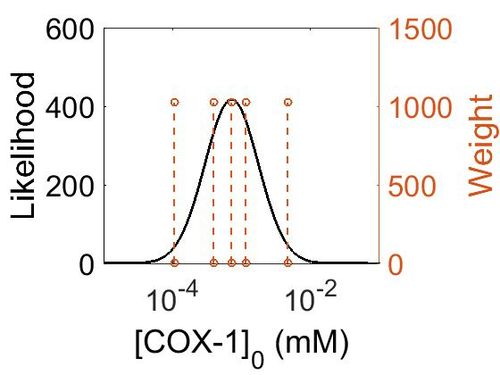
Enzyme concentration
To convert the enzyme concentration from ppm to mM, the following equation was used.
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 849 | 
|
Human | Expression Vector: Platlet
Enzyme: Cyclooxygenase-1 (PGTS1) pH: 7.5 Temperature: 37 °C |
1024 | [27] |
| 208 | 
|
Human | Expression Vector: Stomach
Enzyme: Cyclooxygenase-1 (PGTS1) pH: 7.5 Temperature: 37 °C |
1024 | [28] |
| 130 | 
|
Human | Expression Vector: Esophagus
Enzyme: Cyclooxygenase-1 (PGTS1) pH: 7.5 Temperature: 37 °C |
1024 | [28] |
| 70.8 | 
|
Human | Expression Vector: Oral Cavity
Enzyme: Cyclooxygenase-1 (PGTS1) pH: 7.5 Temperature: 37 °C |
1024 | [28] |
| 19.1 | 
|
Human | Expression Vector: Lung
Enzyme: Cyclooxygenase-1 (PGTS1) pH: 7.5 Temperature: 37 °C |
1024 | [27] |
| Mode (ppm) | Mode (mM) | Confidence Interval | Location parameter (μ) | Scale parameter (σ) |
|---|---|---|---|---|
| 129 | 7.14E-04 | 1.00E+01 | 6.67E+01 | 8.90E-01 |
References
- ↑ Tsai, Al Wei, C. Baek, H. K. Kulmacz, R. J. Van Wart, H. E., Comparison of peroxidase reaction mechanisms of prostaglandin H synthase-1 containing heme and mangano protoporphyrin IX, J Biol Chem (1997), 272, 8885-94.
- ↑ Kulmacz, R. J. van der Donk, W. A. Tsai, A. L. , Comparison of the properties of prostaglandin H synthase-1 and -2, Prog Lipid Res (2003), 42, 377-404
- ↑ Gurram, B. Zhang, S. Li, M. Li, H. Xie, Y. Cui, H. Du, J. Fan, J. Wang, J. Peng, X. , Celecoxib conjugated fluorescent probe for identification and discrimination of cyclooxygenase-2 enzyme in cancer cells, Anal Chem (2018), 90, 5187-5193.
- ↑ Fletcher, B. S. Kujubu, D. A. Perrin, D. M. Herschman, H. R. , Structure of the mitogen-inducible TIS10 gene and demonstration that the TIS10-encoded protein is a functional prostaglandin G/H synthase, J Biol Chem (1992), 267, 4338-44.
- ↑ Xie, W. L. Chipman, J. G. Robertson, D. L. Erikson, R. L. Simmons, D. L. , Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing, Proc Natl Acad Sci U S A (1991), 88, 2692-6.
- ↑ Ristimaki, A. Garfinkel, S. Wessendorf, J. Maciag, T. Hla, T. , Induction of cyclooxygenase-2 by interleukin-1 alpha. Evidence for post-transcriptional regulation, J Biol Chem (1994), 269, 11769-75.
- ↑ name, title, journal (year)
- ↑ Fu, J. Y. Masferrer, J. L. Seibert, K. Raz, A. Needleman, P., The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes, J Biol Chem (1990), 265, 16737-40.
- ↑ Kujubu, D. A. Reddy, S. T. Fletcher, B. S. Herschman, H. R., Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mitogen-stimulated Swiss 3T3 cells, J Biol Chem (1993), 268, 5425-30.
- ↑ Wang, D. An, S. J. Wang, W. H. McGiff, J. C. Ferreri, N. R., CaR-mediated COX-2 expression in primary cultured mTAL cells, Am J Physiol Renal Physiol (2001), 281, F658-64.)
- ↑ Obermoser, V. Baecker, D. Schuster, C. Braun, V. Kircher, B. Gust, R. , Chlorinated cobalt alkyne complexes derived from acetylsalicylic acid as new specific antitumor agents, Dalton Trans, 47, 4341-4351.
- ↑ Kirkby, N. S. Chan, M. V. Zaiss, A. K. Garcia-Vaz, E. Jiao, J. Berglund, L. M. Verdu, E. F. Ahmetaj-Shala, B. Wallace, J. L. Herschman, H. R. Gomez, M. F. Mitchell, J. A., Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-kappaB and NFAT transcriptional pathways, Proc Natl Acad Sci U S A (2016), 113, 434-9.
- ↑ Gurram, B. Zhang, S. Li, M. Li, H. Xie, Y. Cui, H. Du, J. Fan, J. Wang, J. Peng, X. , Celecoxib conjugated fluorescent probe for identification and discrimination of cyclooxygenase-2 enzyme in cancer cells, Anal Chem (2018), 90, 5187-5193.
- ↑ Raj, V. Bhadauria, A. S. Singh, A. K. Kumar, U. Rai, A. Keshari, A. K. Kumar, P. Kumar, D. Maity, B. Nath, S. Prakash, A. Ansari, K. M. Jat, J. L. Saha, S. , Novel 1,3,4-thiadiazoles inhibit colorectal cancer via blockade of IL-6/COX-2 mediated JAK2/STAT3 signals as evidenced through data-based mathematical modeling, Cell, 135, 216-26.
- ↑ Smith, W. L. DeWitt, D. L. Garavito, R. M., Cyclooxygenases: structural, cellular, and molecular biology, Annu Rev Biochem (2000), 69, 145-82.
- ↑ van der Donk, W. A. Tsai, A. L. Kulmacz, R. J., The cyclooxygenase reaction mechanism, Biochemistry, 41, 15451-8.
- ↑ Rouzer, C. A. Marnett, L. J., Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases, Chem Rev, 103, 2239-304.
- ↑ Xiao, G. Chen, W. Kulmacz, R. J., Comparison of structural stabilities of prostaglandin H synthase-1 and -2, J Biol Chem (1998), 273, 6801-11.
- ↑ Miyata, A. Yokoyama, C. Ihara, H. Bandoh, S. Takeda, O. Takahashi, E. Tanabe, T., Characterization of the human gene (TBXAS1) encoding thromboxane synthase, Eur J Biochem (1994), 224, 273-9.
- ↑ Ullrich, V. Zou, M. H. Bachschmid, M. , New physiological and pathophysiological aspects on the thromboxane A(2)-prostacyclin regulatory system, Biochim Biophys Acta (2001), 1532, 1-14.
- ↑ Smyth, E. M. Grosser, T. Wang, M. Yu, Y. FitzGerald, G. A., Prostanoids in health and disease, J Lipid Res, 50 Suppl, S423-8.
- ↑ Smith, W. L. DeWitt, D. L. Garavito, R. M., Cyclooxygenases: structural, cellular, and molecular biology, Annu Rev Biochem (2000), 69, 145-82.
- ↑ Ueno, N. Murakami, M. Tanioka, T. Fujimori, K. Tanabe, T. Urade, Y. Kudo, I. , Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2, J Biol Chem (2001), 276, 34918-27.
- ↑ P. Silva, "A theoretical study of radical-only and combined radical/carbocationic mechanisms of arachidonic acid cyclooxygenation by prostaglandin H synthase" Theor Chem Acc (2003) 110: 345
- ↑ Y. Noreen Development of a Radiochemical Cyclooxygenase-1 and -2 in Vitro Assay for Identification of Natural Products as Inhibitors of Prostaglandin BiosynthesisJ. Nat. Prod., 1998, 61 (1), pp 2–7)
- ↑ 26.0 26.1 Mukherjee Molecular Oxygen Dependent Steps in Fatty Acid Oxidation by Cyclooxygenase-1 Biochemistry, 2007, 46 (13), pp 3975–3989
- ↑ 27.0 27.1 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ 28.0 28.1 28.2 [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587]