Difference between revisions of "Transformation of PL to AA"
(→cPLA2a Parameters) |
(→cPLA2a Parameters) |
||
| Line 148: | Line 148: | ||
|<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
|- | |- | ||
| + | |} | ||
| + | |||
| + | {|class="wikitable sortable" | ||
| + | |+ style="text-align: left;" | Gibbs Free Energy Change | ||
| + | |- | ||
| + | ! Value | ||
| + | ! Units | ||
| + | ! Species | ||
| + | ! Notes | ||
| + | ! Reference | ||
| + | |- | ||
| + | |(-13.03833) | ||
| + | |kcal/mol | ||
| + | |Not stated | ||
| + | |Estimated | ||
| + | Enzyme: cPLA2 | ||
| + | Substrate: Phosphatidylcholine | ||
| + | Product: 1-acyl-sn-glycero-3-phosphocholine and a long-chain fatty acid | ||
| + | pH: 7.3 | ||
| + | ionic strength: 0.25 | ||
| + | |<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=PHOSPHOLIPASE-A2-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | ||
|} | |} | ||
Revision as of 15:29, 18 August 2016
Contents
Reaction
Catalysed by cPLA2α (3.1.1.4).
Chemical equation
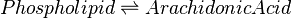
Rate equation
cPLA2a Parameters
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 2.50E-03 | 
|
Mouse | Fibroblasts Cytosolic PLA2 | [1] |
| 5.00E-02 | 
|
Human | Recombinant PLA2 (Phosphatidylcholine as the substrate) | [2] |
| 2.00E-02 | 
|
Human | Recombinant PLA2 (Phosphatidylethanolamine as the substrate) | [2] |
| 4.13E-01 ± 2.80E-02 | 
|
Rat | PMN Neutrophils | [3] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 2.6E+03± 0.1E+03 | 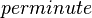
|
Bos taurus | Organism: Bovine
Expression Vector: Bovine pancreatic PLA2 pH: 7.5 Temperature:25'C
|
[4] |
| 3000 | per minute | Porcine | Organism: Porcine
Expression Vector: Porcine Enzme: Pancreatic phospholipase A, Substrate: 1,2-dipalmitoyl-phosphatidylcholine, Enzyme concentration: 0.2 mg/ml, pH: 8.0. Temperature: 41 C |
[5] |
| 11220 | per minute | Stingray | Expression Vector: Stingray
Enzyme: Secreted pH:8.5 Temperature: 40 °C |
[6] |
| 8400 | per minute | Camel | Expression Vector: Camel
Enzyme: Pancreatic pH: 8.5 Temperature: 40 °C |
[6] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 8.26 | 
|
Human | Expression Vector: Lung
Enzyme: cPLA2 pH: 7.5 Temperature: 37 °C |
[7] |
| 42.8 | 
|
Human | Expression Vector: Uterine cervix
Enzyme: 5-LOX pH: 7.5 Temperature: 37 °C |
[8] |
| 111 | 
|
Human | Expression Vector: Oral Cavity
Enzyme: cPLA2 pH: 7.5 Temperature: 37 °C |
[8] |
| 30.8 | 
|
Human | Expression Vector: Liver
Enzyme: cPLA2 pH: 7.5 Temperature: 37 °C |
[8] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| (-13.03833) | kcal/mol | Not stated | Estimated
Enzyme: cPLA2 Substrate: Phosphatidylcholine Product: 1-acyl-sn-glycero-3-phosphocholine and a long-chain fatty acid pH: 7.3 ionic strength: 0.25 |
[9] |
References
- ↑ Spaargaren M. “Characterization and identification of an epidermal-growth-factor-activated phospholipase A2.” Biochem J. 1992 Oct 1; 287(Pt 1): 37–43.
- ↑ 2.0 2.1 Kawauchi Y. “Preparation and characterization of human rheumatoid arthritic synovial fluid phospholipase A2 produced by recombinant baculovirus-infected insect cells.” J Biochem. 1994 Jul;116(1):81-7.
- ↑ Kawauchi Y. “Changes in kinetic properties of cytosolic phospholipase A2 in activated rat neutrophils.” Adv Exp Med Biol. 1997;433:439-42.
- ↑ [www.ncbi.nlm.nih.gov/pubmed/8454568 Hada S. “Hydrolysis of Micellar Diheptanoylphosphatidylcholine Catalyzed byBovine Pancreatic Phospholipase A2: Kinetic Characterization of Group I and II Enzymes1” J Biochem (1993) 113 (1): 13-17.]
- ↑ [http://www.jbc.org/content/261/12/5328.long Menashe M. “Hydrolysis of Dipalmitoylphosphatidylcholine Small Unilamellar Vesicles by Porcine Pancreatic Phospholipase A2” J Biochem (1986) 261, 5328-5333.]
- ↑ 6.0 6.1 Bacha B. “Purification and biochemical characterization of pancreatic phospholipase A2 from the common stingray Dasyatis pastinaca” Lipids Health Dis. 10, 32 (2011)
- ↑ M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ 8.0 8.1 8.2 M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471