Difference between revisions of "Transformation of AA to PGH2"
(→COX-2 Enzyme Parameters) |
(→COX-2 Enzyme Parameters) |
||
| Line 31: | Line 31: | ||
|mM | |mM | ||
|Mouse | |Mouse | ||
| − | |In vitro, | + | |In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. |
|<ref name="Vecchoi2010"> [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]</ref> | |<ref name="Vecchoi2010"> [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]</ref> | ||
|- | |- | ||
| Line 80: | Line 80: | ||
|mM | |mM | ||
|Mouse | |Mouse | ||
| − | |In vitro, | + | |In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. |
|<ref name="Vecchoi2010"> [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]</ref> | |<ref name="Vecchoi2010"> [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]</ref> | ||
|- | |- | ||
| Line 114: | Line 114: | ||
|mM | |mM | ||
|Mouse | |Mouse | ||
| − | |In vitro, | + | |In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. |
|<ref name="Vecchoi2010"> [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]</ref> | |<ref name="Vecchoi2010"> [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]</ref> | ||
|- | |- | ||
Revision as of 16:35, 11 April 2016
Contents
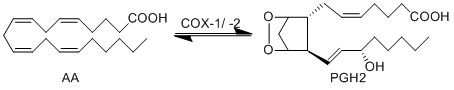
Reaction
Chemical equation
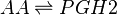
Rate equation
COX-2 Enzyme Parameters
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 1.62E-02 | 
|
Human | X | [1] |
| 5.14E-03 ± 2.90E-04 | mM | Mouse | In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. | [2] |
| 2.1E-03 ± 4.00E-04 | mM | Human | Wild Type Enzyme Assay, 30 °C | [3] |
| 2.1E-03 ± 4.00E-04 | mM | Human | Wild Type cycloxygenase activty, 100 �M arachidonate substrate, pH 7.2. | [4] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 1620 ± 24 | per minute | Mouse | In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. | [2] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 9.45E-03 ± 7.30E-04 | mM | Mouse | In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. | [2] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 522 ± 12.6 | per minute | Mouse | In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. | [2] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 3.68E-02 ± 4.25E-03 | mM | Mouse | In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. | [2] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 207 ± 9 | per minute | Mouse | In vitro, 37 °C, pH 8.0, 1–200 µM of substrate. | [2] |
References
- ↑ S.F. Kim Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science 2005,310(5756):1966-70)
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 [www.ncbi.nlm.nih.gov/pubmed/20463020 Vecchio A. J. "Structural basis of fatty acid substrate binding to cyclooxygenase-2." J. Biol. Chem. 285 22152-63 (2010)]
- ↑ [http://pubs.acs.org/doi/pdf/10.1021/bi035717o Rogge C. "Identification of Tyr504 as an Alternative Tyrosyl Radical Site in Human Prostaglandin H Synthase-2" Biochemistry 2004, 43, 1560-1568]
- ↑ [http://www.jbc.org/content/279/6/4084.full.pdf Bambai B. "Role of Asn-382 and Thr-383 in Activation and Inactivation of Human Prostaglandin H Synthase Cyclooxygenase Catalysis" February 6, 2004 The Journal of Biological Chemistry, 279, 4084-4092.]