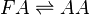Return to overview
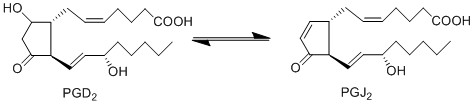
Due to the lack of PGD2 production by keratinocyte and fibroblast cells, the anti-inflammatory cyclopentanones, PGJ2 and 15d-PGJ2, will not be produced during early experimental procedures either.
Reaction
Chemical equation
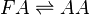
Rate equation
Parameters
Association Rate Constant (Kf)
| Value
|
Units
|
Conditions
|
Substrate
|
Reference
|
| 3.3E+6
|
M-1 min-1
|
25°C and 0.055 ionic strength
|
H2CO3 to CO2
|
[1]
|
Dissociation Rate Constant (Kr)
| Value
|
Units
|
Conditions
|
Substrate
|
Reference
|
|
|
|
|
|
|
Dissociation Constant
| Value
|
Units
|
Conditions
|
Substrate
|
Reference
|
| < 8E-04
|
N/A
|
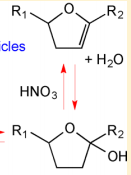
OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2)
|
Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA
|
[2]
|
| 4E-04
|
N/A
|
PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc.
|
H2CO3
|
[3]
|
| 4.4E-4
|
N/A
|
In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3
|
H2CO3
|
[4]
|
Related Reactions