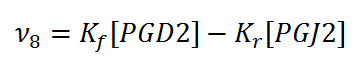Difference between revisions of "Transformation of PGD2 to PGJ2"
(→Parameters) |
|||
| Line 24: | Line 24: | ||
! Conditions | ! Conditions | ||
! Substrate | ! Substrate | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 30: | Line 31: | ||
|25°C and 0.055 ionic strength | |25°C and 0.055 ionic strength | ||
|H2CO3 to CO2 | |H2CO3 to CO2 | ||
| + | |32 | ||
|<ref name="Gibbons1963”>[http://www.jbc.org/content/238/10/3502.full.pdf B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7]</ref> | |<ref name="Gibbons1963”>[http://www.jbc.org/content/238/10/3502.full.pdf B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7]</ref> | ||
|- | |- | ||
| Line 36: | Line 38: | ||
|20°C | |20°C | ||
|HC(OH)2COOH | |HC(OH)2COOH | ||
| + | |32 | ||
|<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | |<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | ||
|- | |- | ||
| Line 42: | Line 45: | ||
|20°C | |20°C | ||
|HC(OH)2COO– | |HC(OH)2COO– | ||
| + | |32 | ||
|<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | |<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | ||
|- | |- | ||
| Line 76: | Line 80: | ||
! Conditions | ! Conditions | ||
! Substrate | ! Substrate | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 83: | Line 88: | ||
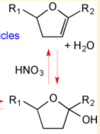
|Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | |Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | ||
[[File:Cyclic Hemiacetals.PNG |center|100px]] | [[File:Cyclic Hemiacetals.PNG |center|100px]] | ||
| + | |8 | ||
|<ref name="Ranney2016”>[http://pubs.acs.org/doi/pdf/10.1021/acs.jpca.6b01402 A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568]</ref> | |<ref name="Ranney2016”>[http://pubs.acs.org/doi/pdf/10.1021/acs.jpca.6b01402 A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568]</ref> | ||
|- | |- | ||
| Line 89: | Line 95: | ||
|PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | |PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | ||
|H2CO3 | |H2CO3 | ||
| + | |8 | ||
|<ref name="BUYTENDYK1927”>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251954/pdf/biochemj01144-0128.pdf F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.]</ref> | |<ref name="BUYTENDYK1927”>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251954/pdf/biochemj01144-0128.pdf F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.]</ref> | ||
|- | |- | ||
| Line 95: | Line 102: | ||
|In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | |In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | ||
|H2CO3 | |H2CO3 | ||
| + | |8 | ||
|<ref name="Thiel1914”>[http://onlinelibrary.wiley.com/doi/10.1002/cber.191404701173/epdf Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.]</ref> | |<ref name="Thiel1914”>[http://onlinelibrary.wiley.com/doi/10.1002/cber.191404701173/epdf Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.]</ref> | ||
|- | |- | ||
Revision as of 10:47, 22 May 2019
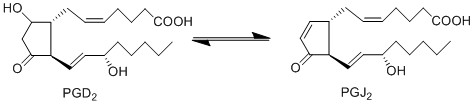
Due to the lack of PGD2 production by keratinocyte and fibroblast cells, the anti-inflammatory cyclopentanones, PGJ2 and 15d-PGJ2, will not be produced during early experimental procedures either.
Contents
Reaction
Chemical equation
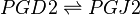
Rate equation
Parameters
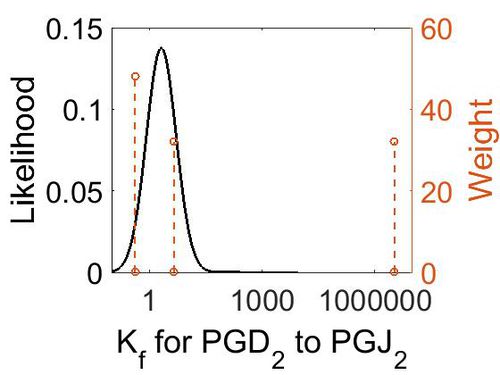
Association Rate Constant (Kf)
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| 3.3E+6 (excluded) | M-1 min-1 | 25°C and 0.055 ionic strength | H2CO3 to CO2 | 32 | [1] |
| 4.5 | M-1 min-1 | 20°C | HC(OH)2COOH | 32 | [2] |
| 0.42 | M-1 min-1 | 20°C | HC(OH)2COO– | 32 | [2] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.48E+00 | 1.09E+03 | 7.34E+00 | 2.42E+00 |
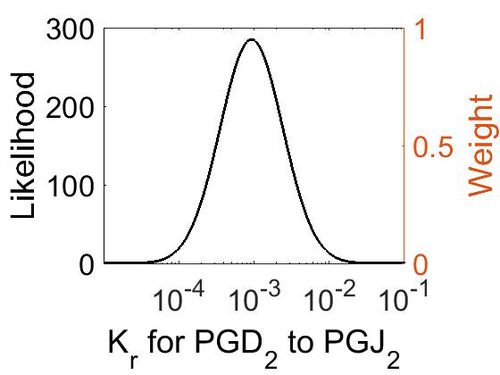
Dissociation Rate Constant (Kr)
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (M-1 s-1) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 9.25E-04 | -6.07E+00 | 9.57E-01 |
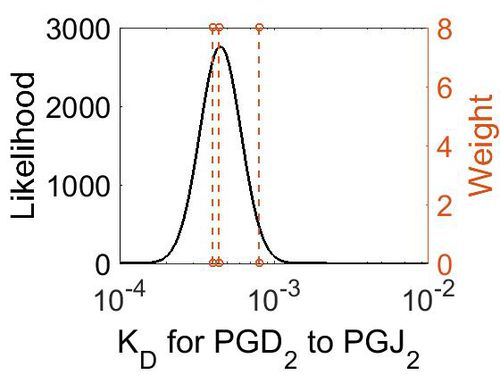
Dissociation Constant
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| < 8E-04 | N/A | OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2) | Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | 8 | [3] |
| 4E-04 | N/A | PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | H2CO3 | 8 | [4] |
| 4.4E-4 | N/A | In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | H2CO3 | 8 | [5] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.49E-04 | 1.38E+00 | -7.61E+00 | 3.07E-01 |
Related Reactions
References
- ↑ B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7
- ↑ 2.0 2.1 [hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]
- ↑ A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568
- ↑ F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.
- ↑ Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.