Difference between revisions of "Transformation of PGD2 to PGJ2"
(→Parameters) |
|||
| Line 64: | Line 64: | ||
|OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2) | |OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2) | ||
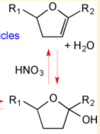
|Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | |Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | ||
| − | [[File:Cyclic Hemiacetals.PNG |center| | + | [[File:Cyclic Hemiacetals.PNG |center|100px]] |
|<ref name="Ranney2016”>[http://pubs.acs.org/doi/pdf/10.1021/acs.jpca.6b01402 A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568]</ref> | |<ref name="Ranney2016”>[http://pubs.acs.org/doi/pdf/10.1021/acs.jpca.6b01402 A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568]</ref> | ||
|- | |- | ||
| Line 83: | Line 83: | ||
== Related Reactions == | == Related Reactions == | ||
* [[Transformation of PGJ2 to 15-D-PGJ2 |Transformation of PGJ2 to 15-D-PGJ2]] | * [[Transformation of PGJ2 to 15-D-PGJ2 |Transformation of PGJ2 to 15-D-PGJ2]] | ||
| + | |||
| + | == References == | ||
| + | <references/> | ||
Revision as of 11:57, 3 April 2017
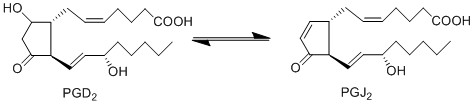
Due to the lack of PGD2 production by keratinocyte and fibroblast cells, the anti-inflammatory cyclopentanones, PGJ2 and 15d-PGJ2, will not be produced during early experimental procedures either.
Contents
Reaction
Chemical equation
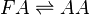
Rate equation
Parameters
| Value | Units | Conditions | Substrate | Reference |
|---|---|---|---|---|
| 3.3E+6 | M-1 min-1 | 25°C and 0.055 ionic strength | H2CO3 to CO2 | [1] |
| Value | Units | Conditions | Substrate | Reference |
|---|---|---|---|---|
| Value | Units | Conditions | Substrate | Reference |
|---|---|---|---|---|
| < 8E-04 | N/A | OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2) | Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | [2] |
| 4E-04 | N/A | PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | H2CO3 | [3] |
| 4.4E-4 | N/A | In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | H2CO3 | [4] |
Related Reactions
References
- ↑ B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7
- ↑ A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568
- ↑ F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.
- ↑ Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.