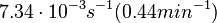Difference between revisions of "Binding of R2 to OA operator"
(→Parameters) |
(→Parameters) |
||
| Line 56: | Line 56: | ||
[[Image:K1-text.png|center|thumb|400px|Li et al. 2012<ref name="Li2012"></ref>]] | [[Image:K1-text.png|center|thumb|400px|Li et al. 2012<ref name="Li2012"></ref>]] | ||
| − | As less information was available for <math>k^{-}_{2}</math> which was acquired by ''in vitro'' measurements and none was derived from ''Streptomyces'', a slightly wider range of values than the ones reported in literature is defined, in order to account for these uncertainty factors. | + | As less information was available for <math>k^{-}_{2}</math>, most of which was acquired by ''in vitro'' measurements and none was derived from ''Streptomyces'', a slightly wider range of values than the ones reported in literature is defined, in order to account for these uncertainty factors. |
|} | |} | ||
Revision as of 07:28, 6 December 2015
The ScbR homo-dimer (R2) binds to the ScbA gene operator (OA) and represses its mRNA transcription.
Contents
Chemical equation
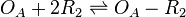
Rate equation
![r= \frac{k^{-}_{2}}{K_{d2}}\cdot [O_{A}]\cdot [R_{2}]^{2} - k^{-}_{2}\cdot [O_{A}-R_{2}]](/wiki/images/math/4/8/5/48594eae1121e765c17117c9d77e08da.png)
Parameters
The parameters of this reaction are the dissociation constant for binding of ScbR to OA (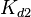 ) and the dissociation rate for binding of ScbR to OA (
) and the dissociation rate for binding of ScbR to OA (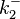 ). ScbR is a member of the TetR family of repressors, named after the member of this group which is the most completely characterized, the TetR repressor protein. TetR binds to the operator tetO, repressing its own expression and that of the efflux determinant tetA in a similar way as ScbR binding to OR and OA and repressing its own expression and the expression of ScbA. Therefore parameter values were derived from published data on the TetR-tetO interaction and on tetR-like proteins binding to their corresponding operators.
). ScbR is a member of the TetR family of repressors, named after the member of this group which is the most completely characterized, the TetR repressor protein. TetR binds to the operator tetO, repressing its own expression and that of the efflux determinant tetA in a similar way as ScbR binding to OR and OA and repressing its own expression and the expression of ScbA. Therefore parameter values were derived from published data on the TetR-tetO interaction and on tetR-like proteins binding to their corresponding operators.
| Name | Value | Units | Value in previous GBL models [1] [2] | Remarks-Reference |
|---|---|---|---|---|
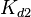
|
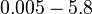 [3] [4] [5] [6] [3] [4] [5] [6]
|
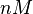
|
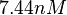
(Range tested: (Bistability range: |
An early publication based on stopped-flow measurements at various salt concentrations reports a KA of 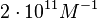 [3], therefore a Kd ( [3], therefore a Kd (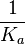 ) of ) of 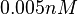 . The Tet repressor and TetO operator were derived by using an overproducing E. coli strain. . The Tet repressor and TetO operator were derived by using an overproducing E. coli strain.
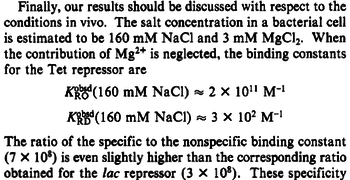 Kleinschmidt et al. 1988[3] Data derived from equilibrium SPR analysis of TetR-tetO interaction report a Ka of 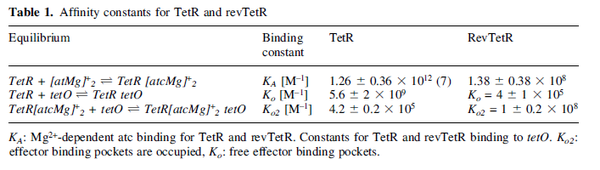 Kamionka et al. 2004 [4] Additionally, a study on the TetR-like protein Rv3066 binding to the mmr operon in M. tuberculosis suggests a Kd of 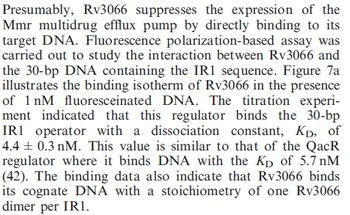 Bolla et al. 2012 [5] Finally, in vitro studies on TetR-like protein ActR in S. coelicolor suggest a Kd in the range of  Ahn et al. 2007 [6] |
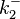
|
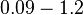 [3] [7] [3] [7]
|

|
N/A | According to the study by Kleinschmidt et al.[3] mentioned above, the maximal association rate constant was 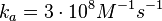 . By taking into account the equilibrium association constant reported above ( . By taking into account the equilibrium association constant reported above (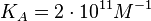 ), the dissociation rate constant can be calculated as per ), the dissociation rate constant can be calculated as per 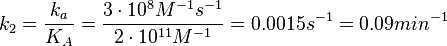 . .
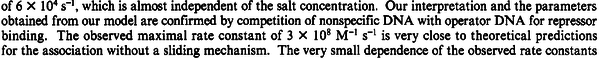 Kleinschmidt et al. 1988[3] Additionally, a study on a TetR-like protein (RolR) which binds to its operator rolO and blocks the transcription of rolHMD and of its own gene, in Corynebacterium glutamicum (Gram positive and GC content ~ 50-60% bacteria) reports the dissociation rates 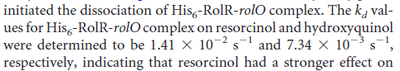 Li et al. 2012[7] As less information was available for |
Parameters with uncertainty
When deciding how to describe the uncertainty for each parameter there are a few points to be taken into consideration. Firstly, the values reported in literature are spread in a relatively large range and correspond to different protein-operator binding reactions, although all are part of the same family of repressors (TetR). Additionally, the values were acquired by in vitro testing, although in some of the publications a strong correlation between in vitro and in vivo measurements is noted. This means that there might be a notable difference between actual parameter values and the ones reported in literature. These facts influence the quantification of the parameter uncertainty and therefore the shape of the corresponding distributions.
More specifically, with regards to the 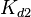 , the value that is considered as the most accurate in different publications is
, the value that is considered as the most accurate in different publications is 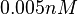 measured by Kleinschmidt et al. However, the Kds measured for the ActR protein in S. coelicolor cover the range of
measured by Kleinschmidt et al. However, the Kds measured for the ActR protein in S. coelicolor cover the range of 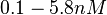 . Therefore, we decided to sample values from the whole range of
. Therefore, we decided to sample values from the whole range of 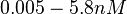 but to keep the weight of the distribution close to the values reported by Kleinschmidt et al. In this context, the mode of the log-normal distribution chosen for the
but to keep the weight of the distribution close to the values reported by Kleinschmidt et al. In this context, the mode of the log-normal distribution chosen for the 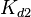 is
is 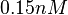 which is within the range of the values reported for S. coelicolor and the confidence interval factor is
which is within the range of the values reported for S. coelicolor and the confidence interval factor is 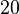 in order to be able to explore a wide range of values and thus take into account the uncertainty caused by both the in vitro testing and the measurements in different species and proteins. Thus, that the range where 95.45% of the values are found is between 0.0075 and 3 nM.
in order to be able to explore a wide range of values and thus take into account the uncertainty caused by both the in vitro testing and the measurements in different species and proteins. Thus, that the range where 95.45% of the values are found is between 0.0075 and 3 nM.
Similarly, the value calculated for the 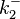 by the measurements of Kleinschmidt et al. is
by the measurements of Kleinschmidt et al. is 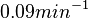 , however other studies reported values of up to
, however other studies reported values of up to 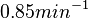 . In order to put the weight towards the mean value but also retain a tendency towards the value reported by Kleinschmidt et al. and also take into account the uncertainty caused by the factors described above, the mode of the log-normal distribution chosen for the
. In order to put the weight towards the mean value but also retain a tendency towards the value reported by Kleinschmidt et al. and also take into account the uncertainty caused by the factors described above, the mode of the log-normal distribution chosen for the 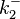 is
is 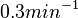 and the confidence interval factor is
and the confidence interval factor is 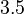 . This means that the range where 95.45% of the values are found is between 0.086 and 1.05
. This means that the range where 95.45% of the values are found is between 0.086 and 1.05  .
.
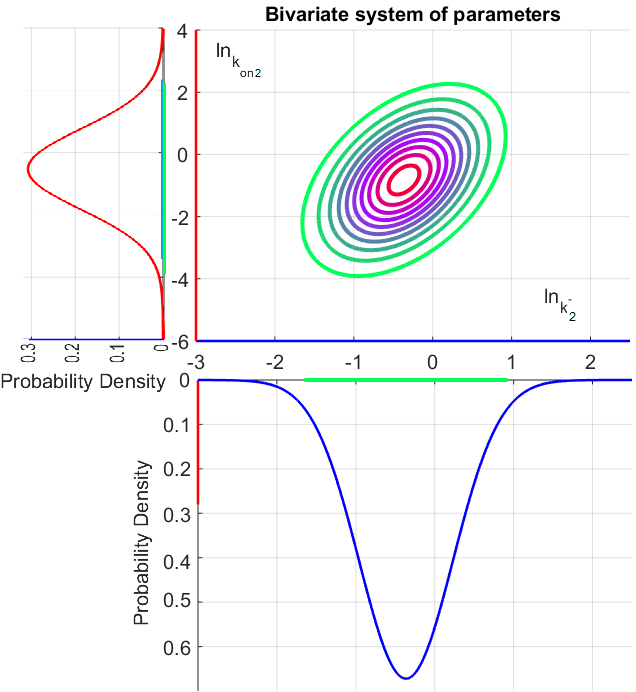
Since the two parameters are interdependent, thermodynamic consistency also needs to be taken into account. This is achieved by creating a bivariate system as described here. Since 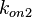 is the parameter with the largest geometric coefficient of variation, as only one reported value was retrieved from literature, this is set as the dependent parameter as per:
is the parameter with the largest geometric coefficient of variation, as only one reported value was retrieved from literature, this is set as the dependent parameter as per: 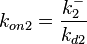 . The location and scale parameters of
. The location and scale parameters of 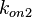 (μ=-0.17013 and σ=1.21377) were calculated from those of
(μ=-0.17013 and σ=1.21377) were calculated from those of 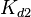 and
and 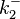 .
.
The probability distributions for the two parameters, adjusted accordingly in order to reflect the above values, are the following:
The correlation matrix which is necessary to define the relationship between the two marginal distributions (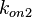 ,
,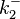 ) of the bivariate system is derived by employing random values generated by the two distributions.
) of the bivariate system is derived by employing random values generated by the two distributions.
The parameters of the distributions of the multivariate system are:
| Parameter | μ | σ | Correlation matrix |
|---|---|---|---|
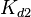
|
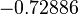
|
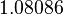
|
N/A |
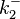
|
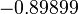
|
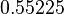
|
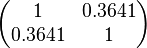
|
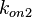
|
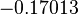
|
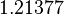
|
The multivariate system of the normal distributions (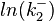 and
and 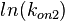 ) and the resulting samples of values are presented in the following figure:
) and the resulting samples of values are presented in the following figure:
In this way, a system of distributions is created where each distribution is described and constrained by the other two. Therefore, the parameters will be sampled by the two marginal distributions in a way consistent with our beliefs and with the relevant thermodynamic constraints. However, since the model's reaction rate requires the parameters 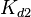 and
and 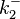 , and not the
, and not the 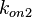 , the value for
, the value for 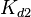 is calculated by the parameters sampled from the other two distributions in an additional step, as per
is calculated by the parameters sampled from the other two distributions in an additional step, as per 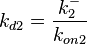 .
.
References
- ↑ S. Mehra, S. Charaniya, E. Takano, and W.-S. Hu. A bistable gene switch for antibiotic biosynthesis: The butyrolactone regulon in streptomyces coelicolor. PLoS ONE, 3(7), 2008.
- ↑ A. Chatterjee, L. Drews, S. Mehra, E. Takano, Y.N. Kaznessis, and W.-S. Hu. Convergent transcription in the butyrolactone regulon in streptomyces coelicolor confers a bistable genetic switch for antibiotic biosynthesis. PLoS ONE, 6(7), 2011.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Kleinschmidt C., Tovar K., Hillen W., Porschke D. Dynamics of repressor-operator recognition: Tn10-encoded tetracycline resistance control. Biochemistry, 1988, 27(4), pp. 1094–1104.
- ↑ 4.0 4.1 4.2 Kamionka A, Bogdanska-Urbaniak J, Scholz O, Hillen W. Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor. Nucleic Acids Research. 2004;32(2):842-847.
- ↑ 5.0 5.1 5.2 Bolla JR, Do SV, Long F, et al. Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis. Nucleic Acids Research. 2012;40(18):9340-9355.
- ↑ 6.0 6.1 6.2 Ahn SK, Tahlan K, Yu Z, Nodwell J. Investigation of Transcription Repression and Small-Molecule Responsiveness by TetR-Like Transcription Factors Using a Heterologous Escherichia coli-Based Assay. Journal of Bacteriology. 2007;189(18):6655-6664.
- ↑ 7.0 7.1 7.2 Li T, Zhao K, Huang Y, et al. The TetR-Type Transcriptional Repressor RolR from Corynebacterium glutamicum Regulates Resorcinol Catabolism by Binding to a Unique Operator, rolO. Applied and Environmental Microbiology. 2012;78(17):6009-6016.


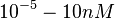 )
)
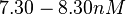 )
)
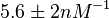
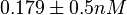 . E. coli was used as a host for the expression of proteins and the experiments were conducted with synthetic tetO-containing fragments.
. E. coli was used as a host for the expression of proteins and the experiments were conducted with synthetic tetO-containing fragments.
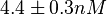 .
.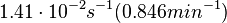 and
and