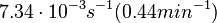Difference between revisions of "Binding of R2 to OA operator"
(→Parameters) |
(→Parameters) |
||
| Line 28: | Line 28: | ||
|<math>0.005 - 5.8 </math> <ref name="Kleinschmidt1988"> [http://pubs.acs.org/doi/pdf/10.1021/bi00404a003 Kleinschmidt C., Tovar K., Hillen W., Porschke D. ''Dynamics of repressor-operator recognition: Tn10-encoded tetracycline resistance control.'' Biochemistry, 1988, 27(4), pp. 1094–1104.]</ref> <ref name="Kamionka2004"> [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC373327/pdf/gkh200.pdf Kamionka A, Bogdanska-Urbaniak J, Scholz O, Hillen W. ''Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor.'' Nucleic Acids Research. 2004;32(2):842-847.]</ref> <ref name="Bolla2012"> [http://nar.oxfordjournals.org/content/40/18/9340.full-text-lowres.pdf Bolla JR, Do SV, Long F, et al. ''Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis.'' Nucleic Acids Research. 2012;40(18):9340-9355.] </ref> <ref name="Ahn2007"> [http://jb.asm.org/content/189/18/6655.full.pdf Ahn SK, Tahlan K, Yu Z, Nodwell J. ''Investigation of Transcription Repression and Small-Molecule Responsiveness by TetR-Like Transcription Factors Using a Heterologous Escherichia coli-Based Assay.'' Journal of Bacteriology. 2007;189(18):6655-6664.]</ref> | |<math>0.005 - 5.8 </math> <ref name="Kleinschmidt1988"> [http://pubs.acs.org/doi/pdf/10.1021/bi00404a003 Kleinschmidt C., Tovar K., Hillen W., Porschke D. ''Dynamics of repressor-operator recognition: Tn10-encoded tetracycline resistance control.'' Biochemistry, 1988, 27(4), pp. 1094–1104.]</ref> <ref name="Kamionka2004"> [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC373327/pdf/gkh200.pdf Kamionka A, Bogdanska-Urbaniak J, Scholz O, Hillen W. ''Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor.'' Nucleic Acids Research. 2004;32(2):842-847.]</ref> <ref name="Bolla2012"> [http://nar.oxfordjournals.org/content/40/18/9340.full-text-lowres.pdf Bolla JR, Do SV, Long F, et al. ''Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis.'' Nucleic Acids Research. 2012;40(18):9340-9355.] </ref> <ref name="Ahn2007"> [http://jb.asm.org/content/189/18/6655.full.pdf Ahn SK, Tahlan K, Yu Z, Nodwell J. ''Investigation of Transcription Repression and Small-Molecule Responsiveness by TetR-Like Transcription Factors Using a Heterologous Escherichia coli-Based Assay.'' Journal of Bacteriology. 2007;189(18):6655-6664.]</ref> | ||
| <math>nM</math> | | <math>nM</math> | ||
| − | | <math> | + | | <math>7.44 nM</math> |
(Range tested: <math>10^{-5}-10 nM</math>) | (Range tested: <math>10^{-5}-10 nM</math>) | ||
| − | (Bistability range: <math> | + | (Bistability range: <math>7.30-8.30 nM</math>) |
|An early publication based on stopped-flow measurements at various salt concentrations reports a K<sub>A</sub> of <math>2 \cdot 10^{11} M^{-1}</math> <ref name="Kleinschmidt1988"></ref>, therefore a K<sub>d</sub> (<math>\frac{1}{K_a}</math>) of <math>0.005 nM </math>. | |An early publication based on stopped-flow measurements at various salt concentrations reports a K<sub>A</sub> of <math>2 \cdot 10^{11} M^{-1}</math> <ref name="Kleinschmidt1988"></ref>, therefore a K<sub>d</sub> (<math>\frac{1}{K_a}</math>) of <math>0.005 nM </math>. | ||
[[Image:Kd1-text3.png|center|thumb|350px|Kleinschmidt et al. 1988<ref name="Kleinschmidt1988"></ref>]] | [[Image:Kd1-text3.png|center|thumb|350px|Kleinschmidt et al. 1988<ref name="Kleinschmidt1988"></ref>]] | ||
Revision as of 04:55, 11 October 2015
The ScbR homo-dimer (R2) binds to the ScbA gene operator (OA) and represses its mRNA transcription.
Contents
Chemical equation
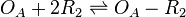
Rate equation
![r= \frac{k^{-}_{2}}{K_{d2}}\cdot [O_{A}]\cdot [R_{2}]^{2} - k^{-}_{2}\cdot [O_{A}-R_{2}]](/wiki/images/math/4/8/5/48594eae1121e765c17117c9d77e08da.png)
Parameters
The parameters of this reaction are the dissociation constant for binding of ScbR to OA (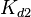 ) and the dissociation rate for binding of ScbR to OA (
) and the dissociation rate for binding of ScbR to OA (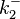 ). ScbR is a member of the TetR family of repressors, named after the member of this group which is the most completely characterized, the TetR repressor protein. TetR binds to the operator tetO, repressing its own expression and that of the efflux determinant tetA in a similar way as ScbR binding to OR and OA and repressing its own expression and the expression of ScbA. Therefore parameter values were derived from published data on the TetR-tetO interaction and on tetR-like proteins binding to their corresponding operators.
). ScbR is a member of the TetR family of repressors, named after the member of this group which is the most completely characterized, the TetR repressor protein. TetR binds to the operator tetO, repressing its own expression and that of the efflux determinant tetA in a similar way as ScbR binding to OR and OA and repressing its own expression and the expression of ScbA. Therefore parameter values were derived from published data on the TetR-tetO interaction and on tetR-like proteins binding to their corresponding operators.
| Name | Value | Units | Value in previous GBL models [1] [2] | Remarks-Reference |
|---|---|---|---|---|
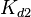
|
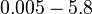 [3] [4] [5] [6] [3] [4] [5] [6]
|
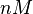
|
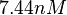
(Range tested: (Bistability range: |
An early publication based on stopped-flow measurements at various salt concentrations reports a KA of 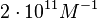 [3], therefore a Kd ( [3], therefore a Kd (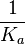 ) of ) of 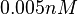 . .
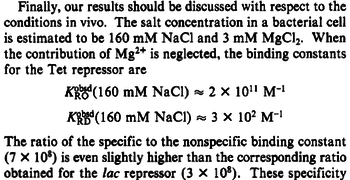 Kleinschmidt et al. 1988[3] Data derived from equilibrium SPR analysis of TetR-tetO interaction report a Ka of 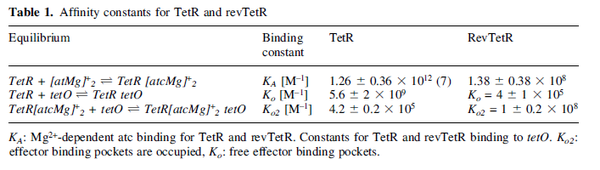 Kamionka et al. 2004 [4] Additionally, a study on the TetR-like protein Rv3066 binding to the mmr operon in M. tuberculosis suggests a Kd of 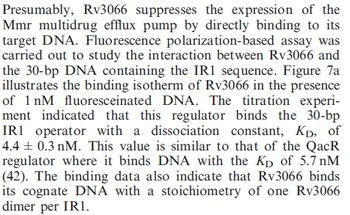 Bolla et al. 2012 [5] Finally, in vitro studies on TetR-like protein ActR in S. coelicolor suggest a Kd in the range of  Ahn et al. 2007 [6] |
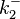
|
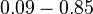 [3] [7] [3] [7]
|

|
N/A | According to the study by Kleinschmidt et al.[3] mentioned above, the maximal association rate constant was 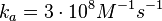 . By taking into account the equilibrium association constant reported above ( . By taking into account the equilibrium association constant reported above (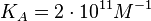 ), the dissociation rate constant can be calculated as per ), the dissociation rate constant can be calculated as per 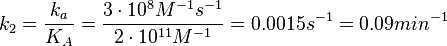 . .
 Kleinschmidt et al. 1988[3] Additionally, a study on a TetR-like protein (RolR) which binds to its operator rolO and blocks the transcription of rolHMD and of its own gene, in Corynebacterium glutamicum (Gram positive and GC content ~ 50-60% bacteria) reports the dissociation rates 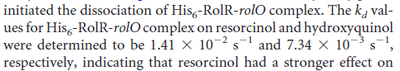 Li et al. 2012[7] |
Parameters with uncertainty
The most plausible parameter value for the 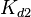 is decided to be
is decided to be 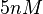 and the confidence interval
and the confidence interval 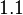 . This means that the mode of the PDF is 5 and the range where 95% of the values are found is between 4.55 and 5.5 nM.
. This means that the mode of the PDF is 5 and the range where 95% of the values are found is between 4.55 and 5.5 nM.
In a similar way, the most plausible value for 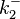 is
is 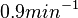 and the confidence interval
and the confidence interval 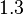 . This means that the mode of the PDF is 0.9 and the range where 95% of the values are found is between 0.6923 and 1.17
. This means that the mode of the PDF is 0.9 and the range where 95% of the values are found is between 0.6923 and 1.17  .
.
The probability distributions for the two parameters, adjusted accordingly in order to reflect the above values, are the following:
The location and scale parameters of the distributions are:
| Parameter | μ | σ |
|---|---|---|
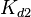
|
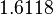
|
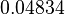
|
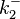
|
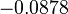
|
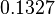
|
References
- ↑ S. Mehra, S. Charaniya, E. Takano, and W.-S. Hu. A bistable gene switch for antibiotic biosynthesis: The butyrolactone regulon in streptomyces coelicolor. PLoS ONE, 3(7), 2008.
- ↑ A. Chatterjee, L. Drews, S. Mehra, E. Takano, Y.N. Kaznessis, and W.-S. Hu. Convergent transcription in the butyrolactone regulon in streptomyces coelicolor confers a bistable genetic switch for antibiotic biosynthesis. PLoS ONE, 6(7), 2011.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Kleinschmidt C., Tovar K., Hillen W., Porschke D. Dynamics of repressor-operator recognition: Tn10-encoded tetracycline resistance control. Biochemistry, 1988, 27(4), pp. 1094–1104.
- ↑ 4.0 4.1 4.2 Kamionka A, Bogdanska-Urbaniak J, Scholz O, Hillen W. Two mutations in the tetracycline repressor change the inducer anhydrotetracycline to a corepressor. Nucleic Acids Research. 2004;32(2):842-847.
- ↑ 5.0 5.1 5.2 Bolla JR, Do SV, Long F, et al. Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis. Nucleic Acids Research. 2012;40(18):9340-9355.
- ↑ 6.0 6.1 6.2 Ahn SK, Tahlan K, Yu Z, Nodwell J. Investigation of Transcription Repression and Small-Molecule Responsiveness by TetR-Like Transcription Factors Using a Heterologous Escherichia coli-Based Assay. Journal of Bacteriology. 2007;189(18):6655-6664.
- ↑ 7.0 7.1 7.2 Li T, Zhao K, Huang Y, et al. The TetR-Type Transcriptional Repressor RolR from Corynebacterium glutamicum Regulates Resorcinol Catabolism by Binding to a Unique Operator, rolO. Applied and Environmental Microbiology. 2012;78(17):6009-6016.


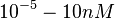 )
)
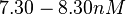 )
)
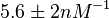
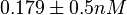 .
.
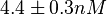 .
.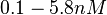
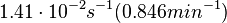 and
and