Difference between revisions of "ATP-Binding Cassette Transporters"
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
| − | + | When a cell produces eicosanoids they are immediately transported into the extracellular compartment, as they are cytotoxic \cite{Pompeia2002}. An ATP-binding cassette (ABC) transporter has been assumed as the method of transportation across the cellular membrane for reactions 22-43. | |
| + | |||
| + | An ABC transporter was assumed as the export transporter of lipids because it is widely reported as transporting organic molecules (ref). Due to the increased amount of free energy provided by the ATP hydrolysis reaction, the transporter is able to relocate molecules against a concentration gradient. The model only contains an inward facing transporter, although in reality there is also an outward facing variant. | ||
| + | |||
| + | In the literature a prostaglandin transporter is reported (Schuster2015), but due to the lack of kinetic analysis of the transporter, the ABC transporter was modelled. We assume that rate of transportation across the membrane may be similar between transporters, however once specific transporter kinetics become available, the model will be updated. | ||
| + | |||
| + | The rate law was designed to encompass the basic principles of an ATP transporter, e.g. the consumption of the internal lipid is directly proportional to amount of transporters. The ratio of substrate and product describes how the gradient would affect the rate of transportation, for example if the concentration of the external lipid equalled that of the internal, transportation would cease. The final term in the equation is the driving force for the transportation, calculated using Gibbs free energy of ATP hydrolysis, the gas constant, temperature and the concentrations of ATP and ADP. As the reformation of ATP is considerably faster than the lipid transporter the ratio was assumed to remain constant. | ||
| − | |||
== Reaction == | == Reaction == | ||
| + | |||
Revision as of 17:38, 4 November 2016
When a cell produces eicosanoids they are immediately transported into the extracellular compartment, as they are cytotoxic \cite{Pompeia2002}. An ATP-binding cassette (ABC) transporter has been assumed as the method of transportation across the cellular membrane for reactions 22-43.
An ABC transporter was assumed as the export transporter of lipids because it is widely reported as transporting organic molecules (ref). Due to the increased amount of free energy provided by the ATP hydrolysis reaction, the transporter is able to relocate molecules against a concentration gradient. The model only contains an inward facing transporter, although in reality there is also an outward facing variant.
In the literature a prostaglandin transporter is reported (Schuster2015), but due to the lack of kinetic analysis of the transporter, the ABC transporter was modelled. We assume that rate of transportation across the membrane may be similar between transporters, however once specific transporter kinetics become available, the model will be updated.
The rate law was designed to encompass the basic principles of an ATP transporter, e.g. the consumption of the internal lipid is directly proportional to amount of transporters. The ratio of substrate and product describes how the gradient would affect the rate of transportation, for example if the concentration of the external lipid equalled that of the internal, transportation would cease. The final term in the equation is the driving force for the transportation, calculated using Gibbs free energy of ATP hydrolysis, the gas constant, temperature and the concentrations of ATP and ADP. As the reformation of ATP is considerably faster than the lipid transporter the ratio was assumed to remain constant.
Contents
Reaction
Chemical equation
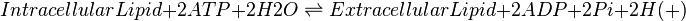
Rate equation
Parameters
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 0.0109 ± 0.00391 | mM | Human | Substrate: LTC4
pH 7.0, 37°C, recombinant MRP2, using 4mM MgATP and 5g of isolated membranes. |
[1] |
| 0.0000366 ± 0.0000038 | mM | Human | Substrate LTC4, 37 °C, 4 mM ATP, | [2] |
| 5.3E-3 ± 2.6E-3 | mM | Human | 4 mM ATP, 37°C, 5–10 ml of membrane vesicle suspension (30
mg protein). |
[3] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 11.34 | per minute | Human | Substrate: ATP | [1] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 0.00019 ± 1.96e-5 | mmol/min/mg protein | Human | Substrate: LTC4
pH 7.0, 37°C, recombinant MRP2, using 4mM MgATP and 5g of isolated membranes. |
[1] |
| 1.25e-7 ± 1.2e-8 | mmol/min/mg | Human | Substrate LTC4, 37 °C, 4 mM ATP, | [2] |
| 2.02e-8 ± 5.9e-9 | mmol/mg/min | Human | 4 mM ATP, 37°C, 5–10 ml of membrane vesicle suspension (30
mg protein). |
[3] |
References
- ↑ 1.0 1.1 1.2 Yasunaga M. "Molecular cloning and functional characterization of cynomolgus monkey multidrug resistance-associated protein 2 (MRP2) Eur. J. Pharm. Sci. 35, 326-334 (2008) Cite error: Invalid
<ref>tag; name "Yas2007" defined multiple times with different content Cite error: Invalid<ref>tag; name "Yas2007" defined multiple times with different content - ↑ 2.0 2.1 Zeng H. "Transport of amphipathic anions by human multidrug resistance protein 3 J. Biol. Chem. 275, 34166-34172 (2000) Cite error: Invalid
<ref>tag; name "Mao2000" defined multiple times with different content - ↑ 3.0 3.1 "Functional reconstitution of substrate transport by purified multidrug resistance protein MRP1 (ABCC1) in phospholipid vesicles Cancer Res. 60, 4779-4784 (2000)