Transformation of LTA4 to LTC4
To generate LTC4, a supramolecular complex of 5-LOX, FLAP and leukotriene C4 synthase is formed on the nuclear membrane due to the increase of intracellular calcium \cite{Woods1993, Hammarberg2000, Radmark2015, Evans2008, Mandal2004, Mandal2008}
Contents
Reaction
Chemical equation
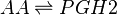
Rate equation
Parameters
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 0.3 �± 0.06 | mM | Human | Expression Vector: E. Coli.
Enzyme: Wild Type hLTC4S pH:7.8 Temperature:20 °C |
[1] |
| 3.00E-02 ± 1.00E-02 | mM | Human | Expression Vector: E Coli
Enzyme: Wild type LTC4S pH: 7.8 Temperature: 37 °C |
[2] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 702 | per minute | Human | Expression Vector: E. Coli.
Enzyme: Wild Type hLTC4S pH:7.8 Temperature:20 °C |
[1] |
| 1560 ± 240 | per minute | Human | Expression Vector: E Coli
Enzyme: Wild type LTC4S pH: 7.8 Temperature: 37 °C |
[2] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 26.8 | 
|
Human | Expression Vector: Lung
Enzyme: LTC4S pH: 7.5 Temperature: 37 °C |
[3] |
| 33.0 | 
|
Human | Expression Vector: Esophagus
Enzyme: LTC4S pH: 7.5 Temperature: 37 °C |
[3] |
| 13.9 | 
|
Human | Expression Vector: Adrenal Gland
Enzyme: LTC4S pH: 7.5 Temperature: 37 °C |
[3] |
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 9.934128 | kcal/mol | Not stated | Estimated
Enzyme: LTC4S Substrate: LTA4 Product: LTC4 pH: 7.3 ionic strength: 0.25 |
[4] |
Related Reactions
| Reaction # | Species | Half Life
(min) |
Rate constant
(min -1) |
Notes | Reference |
|---|---|---|---|---|---|
| 44 | exPGF2a | 528 ± 204 | 0.001 ± 0.003 | Study performed in decidual stromal cells and macrophages in culture. | [5] |
| 45 | exTXB2 | 20 to 30 | 0.035 to 0.023 | Quoted in a textbook(https://books.google.co.uk/books?id=_9kEeTjyJdMC&pg=PA864&lpg=PA864&dq=half+life+txa2&source=bl&ots=2OTF4Mh2Jk&sig=hu79GprliUcW4QE_Zm79islesOA&hl=en&sa=X&ved=0ahUKEwj0oo2sgfjOAhXLIcAKHcaPDHQQ6AEIRjAI#v=onepage&q=half%20life%20txa2&f=false) with no ref. | |
| 46 | exTXA2 | 0.333 | 2.079 | Quoted in a textbook(https://books.google.co.uk/books?id=_9kEeTjyJdMC&pg=PA864&lpg=PA864&dq=half+life+txa2&source=bl&ots=2OTF4Mh2Jk&sig=hu79GprliUcW4QE_Zm79islesOA&hl=en&sa=X&ved=0ahUKEwj0oo2sgfjOAhXLIcAKHcaPDHQQ6AEIRjAI#v=onepage&q=half%20life%20txa2&f=false) with no ref. | |
| 47 | ex6-KETO-PGF2A | 3 | 0.231 | ||
| 48 | exPGI2 | 0.7 | 0.990 | [6] | |
| 49 | exPGE2 | 900,± 492 | 0.001 ± 0.001 | Study performed in decidual stromal cells and macrophages in culture. | [5] |
| 50 | ex15-DEOXY-PGJ2 | 720 | 0.001 | Dehydration of PGD2 to ultimatley 15d-PGJ2 occurs with a half life of about 12 hours in the presense of albumin (protien found in blood). | [7] |
| 51 | exPGJ2 | ||||
| 52 | exPGD2 | 1.5 - 1.6 | 0.462 to 0.433 | Human brain | [8] |
| 53 | exPGH2 | 5 | 0.139 | Quoted on supplier page (http://www.enzolifesciences.com/BML-PH002/prostaglandin-h2/) | |
| 54 | ex5-OXO-ETE | 11 | 0.064 | Study in R15L Cells | [9] |
| 55 | ex5-HETE | ||||
| 56 | exLTB4 | 0.47 ± 0.02 to 0.63 ± 0.04 | 1.475 ± 34.657 to 1.100 ± 17.329 | Rabbit, Immunoreactive LTB4 | [10] |
| 57 | exLTC4 | ||||
| 58 | exLTA4 | 0.05 | 13.863 | 37 degrees C | [11] |
| 59 | ex5-HPETE | ||||
| 60 | ex15-HETE | 21 | 0.0331 | Study in R15L Cells | [9] |
| 61 | ex15-HPETE | ||||
| 62 | ex12-HETE | 180 | 0.004 | "During the first 2 min., the half-life of 12-HETE was 0.9 s, which implies
a fast clearance of the compound from the circulation. However, during the subsequent half-hour the estimated half-life was 3 min. and increased dramatically at the interval of time from 30 to 60 min. (t1/2 around 3 h)." |
[12] |
| 63 | ex12-HPETE | 0.5 | 1.386 | [13] | |
| 64 | exAA | 240 to 660 | 0.003 to 0.001 | [14] |
References
- ↑ 1.0 1.1 [http://www.jbc.org/content/285/52/40771.full.pdf Rinaldo A. " Arginine 104 Is a Key Catalytic Residue in Leukotriene C4 Synthase J Biochem 2010, 285, 40771-40776]
- ↑ 2.0 2.1 [http://www.jbc.org/content/early/2013/12/23/jbc.M113.534628 Niegowski D. " Crystal structures of Leukotriene C4 synthase in complex with product analogs, implications for the
enzyme mechanism J. Biol. Chem. 289, 5199-5207 (2014)] Cite error: Invalid
<ref>tag; name "Niegowski2013" defined multiple times with different content - ↑ 3.0 3.1 3.2 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471
- ↑ 5.0 5.1 O. Ishihara, "Differences of metabolism of prostaglandin E2 and F2 alpha by decidual stromal cells and macrophages in culture." Eicosanoids. 1991;4(4):203-7.
- ↑ Cawello W., "Metabolism and pharmacokinetics of prostaglandin E1 administered by intravenous infusion in human subjects." Eur J Clin Pharmacol. 1994;46(3):275-7.
- ↑ F. Fitzpatrick, "Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro." J Biol Chem. 1983 Oct 10;258(19):11713-8.
- ↑ Suzuki F., "Transport of prostaglandin D2 into brain." Brain Res. 1986 Oct 22;385(2):321-8.
- ↑ 9.0 9.1 Cong W., "15-oxo-Eicosatetraenoic Acid, a Metabolite of Macrophage 15-Hydroxyprostaglandin Dehydrogenase That Inhibits Endothelial Cell Proliferation" Mol Pharmacol. 2009 Sep; 76(3): 516–525.
- ↑ Marleau S., "Metabolic disposition of leukotriene B4 (LTB4) and oxidation-resistant analogues of LTB4 in conscious rabbits." Br J Pharmacol. 1994 Jun;112(2):654-8.
- ↑ Zimmer J., "Fatty acid binding proteins stabilize leukotriene A4 competition with arachidonic acid but not other lipoxygenase products" November 2004 The Journal of Lipid Research, 45, 2138-2144.
- ↑ Dadaian M., "12-hydroxyeicosatetraenoic acid is a long-lived substance in the rabbit circulation." Prostaglandins Other Lipid Mediat. 1998 Jan;55(1):3-25.
- ↑ J. Maclouf, "Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid" (1982) Proc. Natl. Acad. Sci. U. S. A. 79, 6042-6046
- ↑ Vinge E., "Arachidonic acid-induced platelet aggregation and prostanoid formation in whole blood in relation to plasma concentration of indomethacin." Eur J Clin Pharmacol. 1985;28(2):163-9.