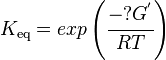Difference between revisions of "Limonene-6-Hydroxylase (L6H)"
Aliah.hawari (talk | contribs) (→Standard Gibbs Free energy) |
Aliah.hawari (talk | contribs) (→Standard Gibbs Free energy) |
||
| Line 145: | Line 145: | ||
==== Standard Gibbs Free energy ==== | ==== Standard Gibbs Free energy ==== | ||
| − | No information yet could be found on the standard Gibbs free energy for L6H. However, Metacyc has the standard Gibbs free energy value for Limonene oxide, catalysing the formation of cis-carveol from limonene [[http://biocyc.org/META/NEW-IMAGE?type=REACTION&object=RXN-9465 RXN-9465]] as '''-92.52051 kcal·mol<sup>-1</sup> ''' <ref name= "Latendresse2013"> | + | No information yet could be found on the standard Gibbs free energy for L6H. However, Metacyc has the standard Gibbs free energy value for Limonene oxide, catalysing the formation of cis-carveol from limonene [[http://biocyc.org/META/NEW-IMAGE?type=REACTION&object=RXN-9465 RXN-9465]] as '''-92.52051 kcal·mol<sup>-1</sup> ''' <ref name= "Latendresse2013"> Latendresse, M. 2013. http://www.biocyc.org/PGDBConceptsGuide.shtml#gibbs. "Computing Gibbs Free Energy of Compounds and Reactions in MetaCyc." </ref>. |
=== Published Kinetic Parameter Values === | === Published Kinetic Parameter Values === | ||
Revision as of 12:16, 17 March 2016
You can go back to main page of the kinetic model here.
Contents
What we know
Issues
Strategies
Reaction catalysed
Metabolite Background Information
Long metabolite names are abbreviated in the model for clarity and standard identification purposes.
| Metabolite | Abbreviation | Chemical Formula | Molar mass (g/mol) | ChEBI | ChEMBL | PubChem |
|---|---|---|---|---|---|---|
| geranyl diphosphate | GPP | C10H20O7P2 | 314.209 | 17211 | 41432 | 445995 |
| (-)-4S-limonene | Limonene | C10H16 | 136.24 | 15384 | 449062 | 22311 or 439250 |
| diphosphate | PP | O7P2 | 173.94 | 644102 |
Equation Rate
| Parameter | Description | Reference |
|---|---|---|
| VLimSynth | Reaction rate for Limonene Synthase | ref |
| Vmaxforward | Maximum reaction rate towards the production of limonene | ref |
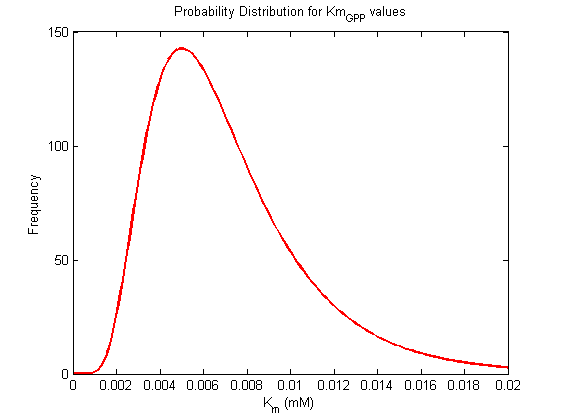
| KmGPP | Michaelis-Menten constant for GPP | ref |
| KmLimonene | Michaelis-Menten constant for Limonene | ref |
| KmPP | Michaelis-Menten constant for PP | ref |
| Keq | Equilibrium constant | ref |
| [GPP] | GPP concentration | ref |
| [Limonene] | Limonene concentration | ref |
| [PP] | PP concentration | ref |
Strategies for estimating the kinetic parameter values
Calculating the Equilibrium Constant
The equilibrium constant can be calculated using the Van't Hoff Isotherm equation:
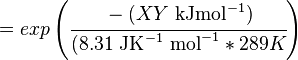
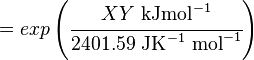
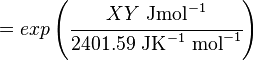
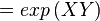
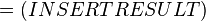
where;
| Keq | Equilibrium constant |
| -?G° | Gibbs free energy change. For (INSERT ENZYME) it is (INSERT VALUE) kJmol-1 |
| R | Gas constant with a value of 8.31 JK-1mol-1 |
| T | Temperature which is always expressed in kelvin |
Standard Gibbs Free energy
No information yet could be found on the standard Gibbs free energy for L6H. However, Metacyc has the standard Gibbs free energy value for Limonene oxide, catalysing the formation of cis-carveol from limonene [RXN-9465] as -92.52051 kcal·mol-1 [1].
Published Kinetic Parameter Values
Km Values
| Km (mM) | Unit | Substrate / Product | Directionality | Organism | References |
|---|---|---|---|---|---|
| Value | unit | substrate | directionality | organism | Ref |
Vmax values
| Vmax | Unit | Directionality | Organism | References |
|---|---|---|---|---|
| Value | µmol/min/mg (unit) | directionality | Organism | References |
Kcat values
| Kcat | Unit | Organism | Reference |
|---|---|---|---|
| value | s-1 | Organism | ref e.g. Alonso 1992 [2] |
Extracting Information from (INSERT SUBSTRATE/PRODUCT) Production Rates
| Amount produced (mg/L) | Time (H) | Organism | Description | Reaction Flux (µM/s) |
|---|---|---|---|---|
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
Published Kinetic Parameter Values
| Km (mM) | Vmax | Kcat (s-1) | Kcat/Km | Organism | Description |
|---|---|---|---|---|---|
| 0.00125 | - | - | Z | A -> B | |
| 0.0018 | - | - | - | Z | A -> B |
| Y | Y | Y | Y | Z | A -> B |
| Y | Y | Y | Y | Z | A -> B |
| Y | Y | - | - | Z | A -> B |
| Y | - | Y | - | Z | GPP -> B |
| Y | - | Y | - | Z | GPP -> B |
| x | - | y | - | Z. | A -> B |
Detailed descriptions of kinetic values used in this model
A more detailed description of the kinetic values listed above can be found here.
Simulations
References
- ↑ Latendresse, M. 2013. http://www.biocyc.org/PGDBConceptsGuide.shtml#gibbs. "Computing Gibbs Free Energy of Compounds and Reactions in MetaCyc."
- ↑ Alonso et. al. 1992. "Purification of 4S-Limonene Synthase, a Monoterpene Cyclase from the Glandular Trichomes of Peppermint (Mentha x piperita) and Spearmint (Mentha spicata)", The Journal of Biological Chemistry, 267(11):7582-7587
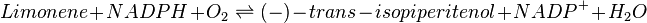
![V_\mathrm{LimSynth} = Vmax_\mathrm{forward} * \cfrac {\cfrac{[GPP]}{Km_\mathrm{GPP}} * \left ( 1 - \cfrac {[Limonene]*[PP]}{[GPP]*K_\mathrm{eq}} \right )}{1 + \cfrac {[GPP]}{Km_\mathrm{GPP}} + \cfrac {[Limonene]}{Km_\mathrm{Limonene}} + \cfrac {[PP]}{Km_\mathrm{PP}} + \cfrac {[Limonene]*[PP]}{Km_\mathrm{Limonene}*Km_\mathrm{PP}}}](/wiki/images/math/6/9/8/698f16353b522504a844aae5c37bdac9.png)