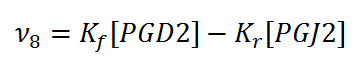Difference between revisions of "Transformation of PGD2 to PGJ2"
(→Parameters) |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
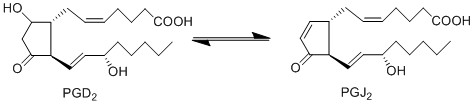
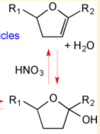
| − | + | PGD2 is subsequently converted to PGJ2 via a non-enzymatic dehydration reaction <ref>Fitzpatrick, F. A. Wynalda, M. A., ''Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro'', J Biol Chem (1983), 258, 11713-8.</ref>. This dehydration reaction takes place in an aqueous solution and includes the removal of two hydrogens and one oxygen molecule. This reaction occurs across the C9 hydroxyl and the C10 carbon axis, to form a double bond between C9 and 10. | |
| Line 12: | Line 12: | ||
== Rate equation == | == Rate equation == | ||
| − | |||
| − | |||
[[File:R08.PNG|center|500px]] | [[File:R08.PNG|center|500px]] | ||
| Line 26: | Line 24: | ||
! Conditions | ! Conditions | ||
! Substrate | ! Substrate | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 32: | Line 31: | ||
|25°C and 0.055 ionic strength | |25°C and 0.055 ionic strength | ||
|H2CO3 to CO2 | |H2CO3 to CO2 | ||
| + | |32 | ||
|<ref name="Gibbons1963”>[http://www.jbc.org/content/238/10/3502.full.pdf B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7]</ref> | |<ref name="Gibbons1963”>[http://www.jbc.org/content/238/10/3502.full.pdf B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7]</ref> | ||
|- | |- | ||
| Line 38: | Line 38: | ||
|20°C | |20°C | ||
|HC(OH)2COOH | |HC(OH)2COOH | ||
| + | |32 | ||
|<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | |<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | ||
|- | |- | ||
| Line 44: | Line 45: | ||
|20°C | |20°C | ||
|HC(OH)2COO– | |HC(OH)2COO– | ||
| + | |32 | ||
|<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | |<ref name="Turyan1998”>[hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]</ref> | ||
|- | |- | ||
| Line 55: | Line 57: | ||
|} | |} | ||
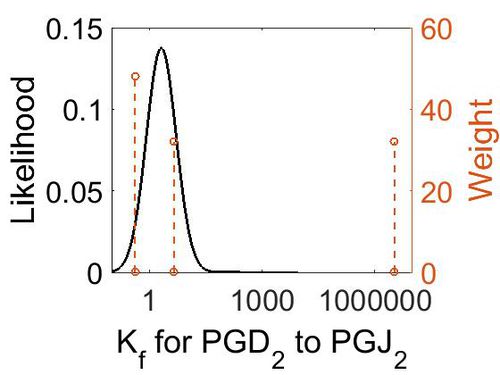
| − | + | [[Image:25.jpg|none|thumb|500px|The estimated probability distribution for reaction 8 Kf. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | |
| Line 67: | Line 69: | ||
|- | |- | ||
|} | |} | ||
| + | |||
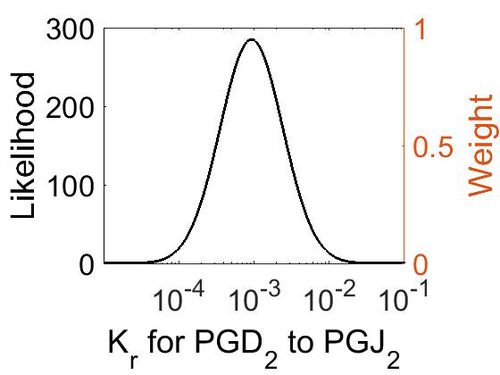
| + | [[Image:26.jpg|none|thumb|500px|The estimated probability distribution for reaction 8 Kr. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
=== Dissociation Constant === | === Dissociation Constant === | ||
| Line 76: | Line 80: | ||
! Conditions | ! Conditions | ||
! Substrate | ! Substrate | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 83: | Line 88: | ||
|Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | |Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | ||
[[File:Cyclic Hemiacetals.PNG |center|100px]] | [[File:Cyclic Hemiacetals.PNG |center|100px]] | ||
| + | |8 | ||
|<ref name="Ranney2016”>[http://pubs.acs.org/doi/pdf/10.1021/acs.jpca.6b01402 A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568]</ref> | |<ref name="Ranney2016”>[http://pubs.acs.org/doi/pdf/10.1021/acs.jpca.6b01402 A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568]</ref> | ||
|- | |- | ||
| Line 89: | Line 95: | ||
|PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | |PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | ||
|H2CO3 | |H2CO3 | ||
| + | |8 | ||
|<ref name="BUYTENDYK1927”>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251954/pdf/biochemj01144-0128.pdf F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.]</ref> | |<ref name="BUYTENDYK1927”>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1251954/pdf/biochemj01144-0128.pdf F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.]</ref> | ||
|- | |- | ||
| Line 95: | Line 102: | ||
|In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | |In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | ||
|H2CO3 | |H2CO3 | ||
| + | |8 | ||
|<ref name="Thiel1914”>[http://onlinelibrary.wiley.com/doi/10.1002/cber.191404701173/epdf Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.]</ref> | |<ref name="Thiel1914”>[http://onlinelibrary.wiley.com/doi/10.1002/cber.191404701173/epdf Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.]</ref> | ||
|- | |- | ||
| Line 106: | Line 114: | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | [[Image:R8 kd.jpg|none|thumb|500px|The estimated probability distribution for reaction 8 KD. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
== Related Reactions == | == Related Reactions == | ||
Latest revision as of 08:09, 21 August 2019
PGD2 is subsequently converted to PGJ2 via a non-enzymatic dehydration reaction [1]. This dehydration reaction takes place in an aqueous solution and includes the removal of two hydrogens and one oxygen molecule. This reaction occurs across the C9 hydroxyl and the C10 carbon axis, to form a double bond between C9 and 10.
Contents
Reaction
Chemical equation
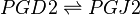
Rate equation
Parameters
Association Rate Constant (Kf)
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| 3.3E+6 (excluded) | M-1 min-1 | 25°C and 0.055 ionic strength | H2CO3 to CO2 | 32 | [2] |
| 4.5 | M-1 min-1 | 20°C | HC(OH)2COOH | 32 | [3] |
| 0.42 | M-1 min-1 | 20°C | HC(OH)2COO– | 32 | [3] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.48E+00 | 1.09E+03 | 7.34E+00 | 2.42E+00 |
Dissociation Rate Constant (Kr)
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (M-1 s-1) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 9.25E-04 | -6.07E+00 | 9.57E-01 |
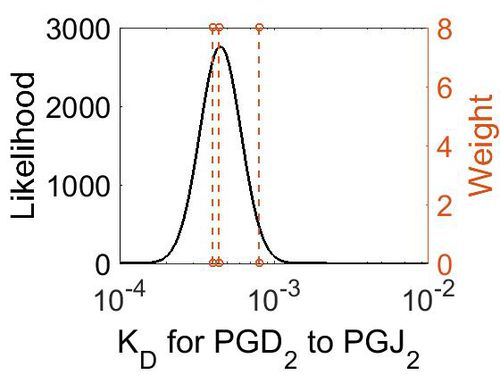
Dissociation Constant
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| < 8E-04 | N/A | OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2) | Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | 8 | [4] |
| 4E-04 | N/A | PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | H2CO3 | 8 | [5] |
| 4.4E-4 | N/A | In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | H2CO3 | 8 | [6] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.49E-04 | 1.38E+00 | -7.61E+00 | 3.07E-01 |
Related Reactions
References
- ↑ Fitzpatrick, F. A. Wynalda, M. A., Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro, J Biol Chem (1983), 258, 11713-8.
- ↑ B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7
- ↑ 3.0 3.1 [hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]
- ↑ A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568
- ↑ F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.
- ↑ Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.