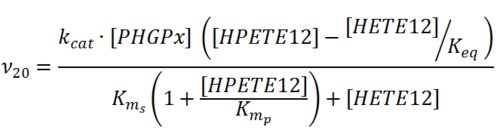Difference between revisions of "Transformation of 12-HPETE to 12-HETE"
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
| + | |||
| + | Upon being generated, 12-HPETE can be reduced by an oxidoreductase enzyme, phospholipid hydroperoxide glutathione peroxidase (PHGPx), to form 12-HETE respectively. | ||
| + | |||
== Reaction == | == Reaction == | ||
| Line 6: | Line 9: | ||
==Chemical equation== | ==Chemical equation== | ||
| − | <center><math> | + | <center><math> 12-HPETE \rightleftharpoons 12-HETE </math></center> |
== Rate equation == | == Rate equation == | ||
| + | [[File:R20.PNG |center|500px]] | ||
== Parameters == | == Parameters == | ||
| + | Note that the literature values are the same as [[Transformation of 5-HPETE to 5-HETE |reaction 12]]. | ||
| + | |||
| + | === K<sub>ms</sub> === | ||
| + | {|class="wikitable sortable" | ||
| + | |+ style="text-align: left;" | Literature values | ||
| + | |- | ||
| + | ! Value | ||
| + | ! Units | ||
| + | ! Species | ||
| + | ! Notes | ||
| + | ! Weight | ||
| + | ! Reference | ||
| + | |- | ||
| + | |3.00E-01 | ||
| + | |<math> mM </math> | ||
| + | |Human | ||
| + | |Expression Vector: Bioimprited Enzyme - Selenosubtilisin | ||
| + | Enzyme: Glutathione Peroxidase | ||
| + | pH: 7 | ||
| + | Temperature: 37 | ||
| + | |512 | ||
| + | |<ref name="Liu2008"> [http://www.ncbi.nlm.nih.gov/pubmed/18163571 Liu L. "Functional mimicry of the active site of glutathione peroxidase by glutathione imprinted selenium-containing protein.'' Biomacromolecules. 2008 Jan;9(1):363-8. doi: 10.1021/bm7008312. Epub 2007 Dec 29.]</ref> | ||
| + | |- | ||
| + | |11.1 ± 2.90E-01 | ||
| + | |<math> mM </math> | ||
| + | |Human | ||
| + | |Expression Vector: E Coli | ||
| + | Enzyme: Wild Type Glutathione Peroxidase Enzyme (Se-hGSTZ1-1) | ||
| + | pH: 7 | ||
| + | Temperature: 37 | ||
| + | |1024 | ||
| + | |<ref name="Zheng2008"> [http://www.ncbi.nlm.nih.gov/pubmed/18373941 Zheng K. "A novel selenium-containing glutathione transferase zeta1-1, the activity of which surpasses the level of some native glutathione peroxidases.'' Int J Biochem Cell Biol. 2008;40(10):2090-7. doi: 10.1016/j.biocel.2008.02.006. Epub 2008 Feb 15.]</ref> | ||
| + | |- | ||
| + | |4.00E-03 | ||
| + | |<math> mM </math> | ||
| + | |Rat | ||
| + | |Expression Vector: Rat Liver Cells | ||
| + | Enzyme: Glutathione Peroxidase | ||
| + | pH: 7.4 | ||
| + | Temperature: 37 | ||
| + | |768 | ||
| + | |<ref name="Hirat1997"> [http://www.ncbi.nlm.nih.gov/pubmed/9030530 Hiratsuka A. "Subunit Ya-specific glutathione peroxidase activity toward cholesterol 7-hydroperoxides of glutathione S-transferases in cytosols from rat liver and skin.'' J Biol Chem. 1997 Feb 21;272(8):4763-9.]</ref> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the PHGPx Kms distribution | ||
| + | ! Mode (mM) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 3.19E-01 || 1.02E+03 || 2.54E+00 || 1.92E+00 | ||
| + | |} | ||
| + | |||
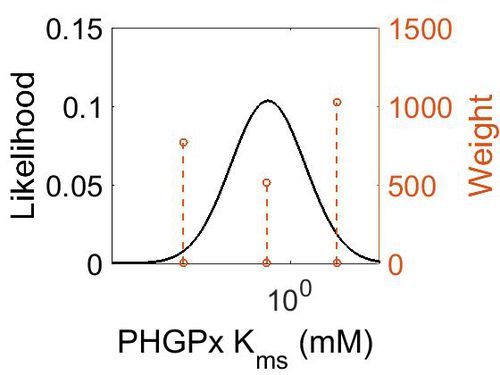
| + | [[Image:37.jpg|none|thumb|500px|The estimated probability distribution for PHGPx Kms. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
| + | === K<sub>mp</sub> === | ||
| + | This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail[[Quantification of parameter uncertainty | here]]). As a result, no confidence interval factor or literature values were cited for this parameter. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the PHGPx Kmp distribution | ||
| + | ! Mode (mM) !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 3.15E-01 || 2.53E+00 || 1.92E+00 | ||
| + | |} | ||
| + | |||
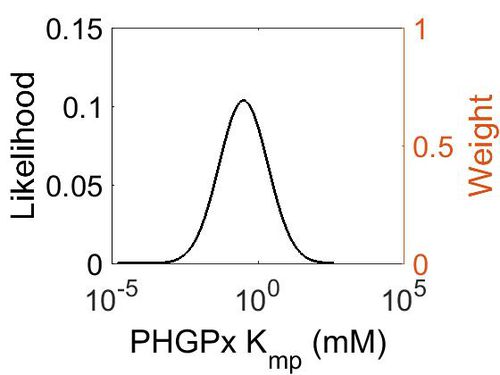
| + | [[Image:38.jpg|none|thumb|500px|The estimated probability distribution for PHGPx Kmp. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
| + | === k<sub>cat</sub> === | ||
| + | {|class="wikitable sortable" | ||
| + | |+ style="text-align: left;" | Literature values | ||
| + | |- | ||
| + | ! Value | ||
| + | ! Units | ||
| + | ! Species | ||
| + | ! Notes | ||
| + | ! Weight | ||
| + | ! Reference | ||
| + | |- | ||
| + | |1170 ± 50 | ||
| + | |<math> min^{-1} </math> | ||
| + | |Human | ||
| + | |Expression Vector: Bioimprited Enzyme - Selenosubtilisin | ||
| + | Enzyme: Glutathione Peroxidase | ||
| + | pH: 7 | ||
| + | Temperature: 37 | ||
| + | | 512 | ||
| + | |<ref name="Liu2008"> [http://www.ncbi.nlm.nih.gov/pubmed/18163571 Liu L. "Functional mimicry of the active site of glutathione peroxidase by glutathione imprinted selenium-containing protein.'' Biomacromolecules. 2008 Jan;9(1):363-8. doi: 10.1021/bm7008312. Epub 2007 Dec 29.]</ref> | ||
| + | |- | ||
| + | |24500 ± 150 | ||
| + | |<math> min^{-1} </math> | ||
| + | |Human | ||
| + | |Expression Vector: E Coli | ||
| + | Enzyme: Wild Type Glutathione Peroxidase Enzyme (Se-hGSTZ1-1) | ||
| + | pH: 7 | ||
| + | Temperature: 37 | ||
| + | |1024 | ||
| + | |<ref name="Zheng2008"> [http://www.ncbi.nlm.nih.gov/pubmed/18373941 Zheng K. "A novel selenium-containing glutathione transferase zeta1-1, the activity of which surpasses the level of some native glutathione peroxidases.'' Int J Biochem Cell Biol. 2008;40(10):2090-7. doi: 10.1016/j.biocel.2008.02.006. Epub 2008 Feb 15.]</ref> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the PHGPx kcat distribution | ||
| + | ! Mode (min-1) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
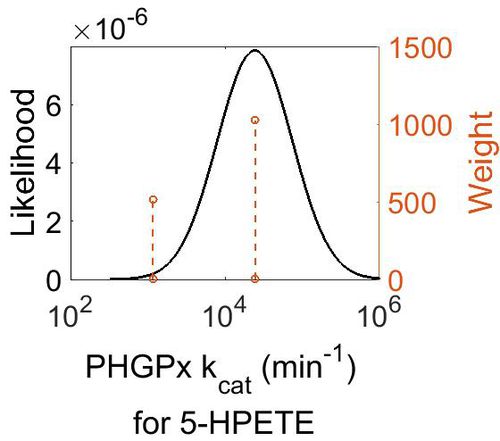
| + | | 2.44E+04 || 4.20E+00 || 1.11E+01 || 9.75E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:39.jpg|none|thumb|500px|The estimated probability distribution for PHGPx kcat. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| − | + | === Enzyme concentration === | |
| − | {|class="wikitable" | + | To convert the enzyme concentration from ppm to mM, the following [[Common equations#Enzyme concentration (mM)|equation]] was used. |
| − | + | ||
| + | {|class="wikitable sortable" | ||
| + | |+ style="text-align: left;" | Literature values | ||
| + | |- | ||
! Value | ! Value | ||
! Units | ! Units | ||
! Species | ! Species | ||
| + | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| − | | | + | |459 |
| − | | | + | |<math> ppm </math> |
| − | | | + | |Human |
| − | | A | + | |Expression Vector: Skin |
| − | | | + | Enzyme: PHGPx |
| + | pH: 7.5 | ||
| + | Temperature: 37 °C | ||
| + | |2048 | ||
| + | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
| + | |- | ||
| + | |307 | ||
| + | |<math> ppm </math> | ||
| + | |Human | ||
| + | |Expression Vector: Lung | ||
| + | Enzyme: PHGPx | ||
| + | pH: 7.5 | ||
| + | Temperature: 37 °C | ||
| + | |1024 | ||
| + | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
| + | |- | ||
| + | |282 | ||
| + | |<math> ppm </math> | ||
| + | |Human | ||
| + | |Expression Vector: Esophagus | ||
| + | Enzyme: PHGPx | ||
| + | pH: 7.5 | ||
| + | Temperature: 37 °C | ||
| + | |1024 | ||
| + | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
| + | |- | ||
| + | |204 | ||
| + | |<math> ppm </math> | ||
| + | |Human | ||
| + | |Expression Vector: Gut | ||
| + | Enzyme: PHGPx | ||
| + | pH: 7.5 | ||
| + | Temperature: 37 °C | ||
| + | |1024 | ||
| + | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
| + | |- | ||
|} | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the PHGPx concentration distribution | ||
| + | ! Mode (ppm) !! Mode (mM) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
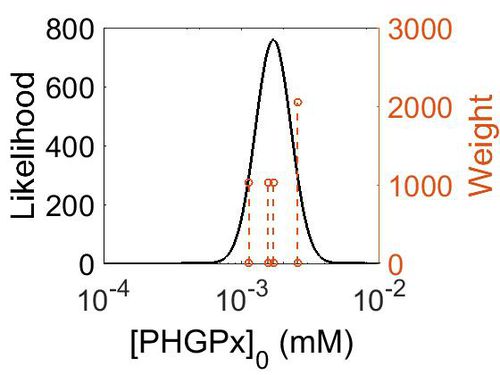
| + | | 3.14E+02 ||1.74E-03|| 1.38E+00 || 5.85E+00 || 3.09E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:166.jpg|none|thumb|500px|The estimated probability distribution for PHGPx concentration. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
| + | === K<sub>eq</sub> === | ||
| + | |||
| + | {|class="wikitable sortable" | ||
| + | |+ style="text-align: left;" | Literature values | ||
| + | |- | ||
| + | ! Gibbs Free Energy Change | ||
| + | ! Units | ||
| + | ! Species | ||
| + | ! Notes | ||
| + | ! Weight | ||
| + | ! Reference | ||
| + | |- | ||
| + | |(-26.941177) | ||
| + | |kcal/mol | ||
| + | |Not stated | ||
| + | |Estimated | ||
| + | Enzyme: PHGPx | ||
| + | Substrate:a hydroperoxy-fatty-acyl-[lipid] | ||
| + | Product: a hydroxy-fatty-acyl-[lipid] | ||
| + | pH: 7.3 | ||
| + | ionic strength: 0.25 | ||
| + | |64 | ||
| + | |<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=1.11.1.12-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the PHGPx Keq distribution | ||
| + | ! Mode !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
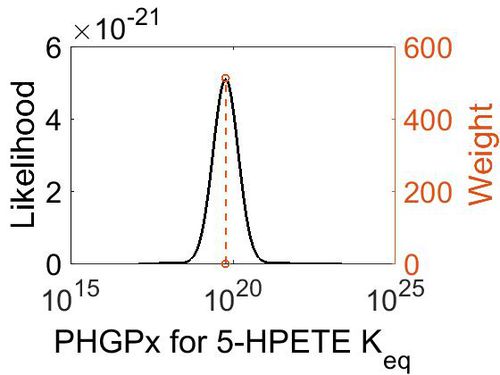
| + | | 5.90E+19 || 1.00E+01 || 4.63E+01 || 8.90E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:40.jpg|none|thumb|500px|The estimated probability distribution for PHGPx Keq. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
== Related Reactions == | == Related Reactions == | ||
Latest revision as of 08:20, 21 August 2019
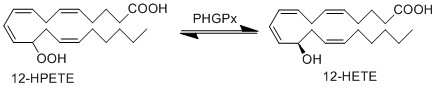
Upon being generated, 12-HPETE can be reduced by an oxidoreductase enzyme, phospholipid hydroperoxide glutathione peroxidase (PHGPx), to form 12-HETE respectively.
Contents
Reaction
Chemical equation
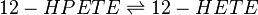
Rate equation
Parameters
Note that the literature values are the same as reaction 12.
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 3.00E-01 | 
|
Human | Expression Vector: Bioimprited Enzyme - Selenosubtilisin
Enzyme: Glutathione Peroxidase pH: 7 Temperature: 37 |
512 | [1] |
| 11.1 ± 2.90E-01 | 
|
Human | Expression Vector: E Coli
Enzyme: Wild Type Glutathione Peroxidase Enzyme (Se-hGSTZ1-1) pH: 7 Temperature: 37 |
1024 | [2] |
| 4.00E-03 | 
|
Rat | Expression Vector: Rat Liver Cells
Enzyme: Glutathione Peroxidase pH: 7.4 Temperature: 37 |
768 | [3] |
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 3.19E-01 | 1.02E+03 | 2.54E+00 | 1.92E+00 |
Kmp
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (mM) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 3.15E-01 | 2.53E+00 | 1.92E+00 |
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 1170 ± 50 | 
|
Human | Expression Vector: Bioimprited Enzyme - Selenosubtilisin
Enzyme: Glutathione Peroxidase pH: 7 Temperature: 37 |
512 | [1] |
| 24500 ± 150 | 
|
Human | Expression Vector: E Coli
Enzyme: Wild Type Glutathione Peroxidase Enzyme (Se-hGSTZ1-1) pH: 7 Temperature: 37 |
1024 | [2] |
| Mode (min-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 2.44E+04 | 4.20E+00 | 1.11E+01 | 9.75E-01 |
Enzyme concentration
To convert the enzyme concentration from ppm to mM, the following equation was used.
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 459 | 
|
Human | Expression Vector: Skin
Enzyme: PHGPx pH: 7.5 Temperature: 37 °C |
2048 | [4] |
| 307 | 
|
Human | Expression Vector: Lung
Enzyme: PHGPx pH: 7.5 Temperature: 37 °C |
1024 | [4] |
| 282 | 
|
Human | Expression Vector: Esophagus
Enzyme: PHGPx pH: 7.5 Temperature: 37 °C |
1024 | [4] |
| 204 | 
|
Human | Expression Vector: Gut
Enzyme: PHGPx pH: 7.5 Temperature: 37 °C |
1024 | [5] |
| Mode (ppm) | Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|---|
| 3.14E+02 | 1.74E-03 | 1.38E+00 | 5.85E+00 | 3.09E-01 |
Keq
| Gibbs Free Energy Change | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| (-26.941177) | kcal/mol | Not stated | Estimated
Enzyme: PHGPx Substrate:a hydroperoxy-fatty-acyl-[lipid] Product: a hydroxy-fatty-acyl-[lipid] pH: 7.3 ionic strength: 0.25 |
64 | [6] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 5.90E+19 | 1.00E+01 | 4.63E+01 | 8.90E-01 |
Related Reactions
- ↑ 1.0 1.1 Liu L. "Functional mimicry of the active site of glutathione peroxidase by glutathione imprinted selenium-containing protein. Biomacromolecules. 2008 Jan;9(1):363-8. doi: 10.1021/bm7008312. Epub 2007 Dec 29.
- ↑ 2.0 2.1 Zheng K. "A novel selenium-containing glutathione transferase zeta1-1, the activity of which surpasses the level of some native glutathione peroxidases. Int J Biochem Cell Biol. 2008;40(10):2090-7. doi: 10.1016/j.biocel.2008.02.006. Epub 2008 Feb 15.
- ↑ Hiratsuka A. "Subunit Ya-specific glutathione peroxidase activity toward cholesterol 7-hydroperoxides of glutathione S-transferases in cytosols from rat liver and skin. J Biol Chem. 1997 Feb 21;272(8):4763-9.
- ↑ 4.0 4.1 4.2 M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587
- ↑ M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471