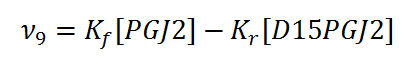Difference between revisions of "Transformation of PGJ2 to 15-D-PGJ2"
(→Parameters) |
|||
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
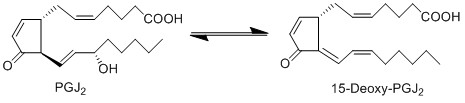
| − | + | In aqueous solutions containing serum albumin, PGJ2 is subject to an additional non-enzymatic dehydration reaction at the hydroxyl group of C15 to yield 15-deoxy-PGJ2. The products of these dehydration reactions have higher activity than the parent compound PGD2 <ref>Straus, D. S. Glass, C. K., ''Cyclopentenone prostaglandins: new insights on biological activities and cellular targets'', Med Res Rev (2001), 21, 185-210.</ref>. | |
== Reaction == | == Reaction == | ||
Latest revision as of 08:11, 21 August 2019
In aqueous solutions containing serum albumin, PGJ2 is subject to an additional non-enzymatic dehydration reaction at the hydroxyl group of C15 to yield 15-deoxy-PGJ2. The products of these dehydration reactions have higher activity than the parent compound PGD2 [1].
Contents
Reaction
Chemical equation
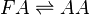
Rate equation
Parameters
Note that the literature values are the same as reaction 8.
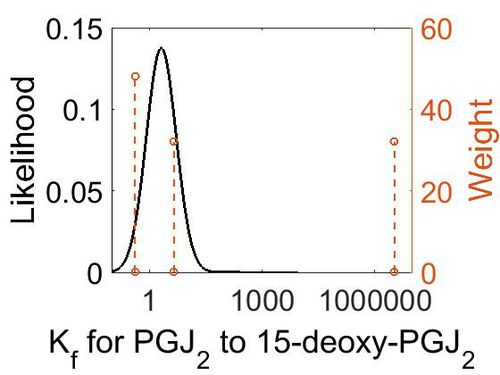
Association Rate Constant (Kf)
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| 3.3E+6 (excluded) | M-1 min-1 | 25°C and 0.055 ionic strength | H2CO3 to CO2 | 32 | [2] |
| 4.5 | M-1 min-1 | 20°C | HC(OH)2COOH | 32 | [3] |
| 0.42 | M-1 min-1 | 20°C | HC(OH)2COO– | 32 | [3] |
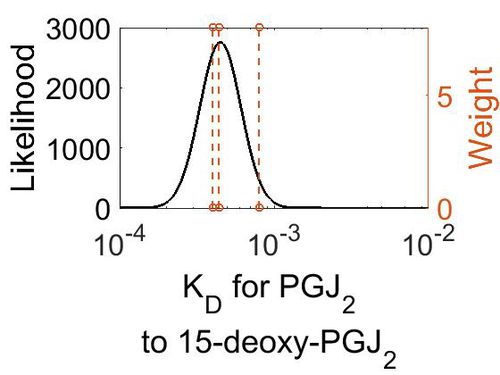
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.48E+00 | 1.09E+03 | 7.34E+00 | 2.42E+00 |
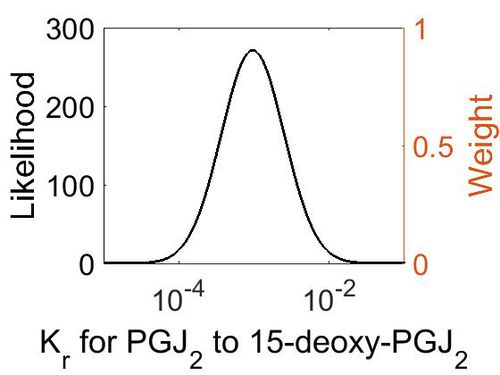
Dissociation Rate Constant (Kr)
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (M-1 s-1) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 9.25E-04 | -6.07E+00 | 9.57E-01 |
Dissociation Constant
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
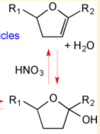
| < 8E-04 | N/A | OH Radicals in the Presence of Added Gas Phase HNO3 (3 ppmv n-pentadecane, 0.25, 0.50, 1.0, or 2.0 ppmv HNO3, 10 ppmv O3, and 2 ppmv TME were added from a glass bulb in a flow of N2) | Acid-Catalyzed Dehydration of Cyclic Hemiacetals (n-Pentadecane) in SOA | 8 | [4] |
| 4E-04 | N/A | PH of 100 cc. 0-02N NaHCO3, saturated with C02, into which 0 95 cc. | H2CO3 | 8 | [5] |
| 4.4E-4 | N/A | In a 0O008 mol. solution of carbonic acid at 4°, 1-23 % is present as H2CO3 | H2CO3 | 8 | [6] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.49E-04 | 1.38E+00 | -7.61E+00 | 3.07E-01 |
Related Reactions
- Transformation of PGD2 to PGJ2
- ↑ Straus, D. S. Glass, C. K., Cyclopentenone prostaglandins: new insights on biological activities and cellular targets, Med Res Rev (2001), 21, 185-210.
- ↑ B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7
- ↑ 3.0 3.1 [hrcak.srce.hr/file/195437 Y.I. Tur'yan, "Kinetics and Equilibrium of the Dehydration-Hydration and Recombination-Dissociation Reactions of Glyoxylic Acid Investigated by Electrochemical Methods", CCACAA 71 (3) 727¿743 (1998)]
- ↑ A. Ranney "Kinetics of Acid-Catalyzed Dehydration of Cyclic Hemiacetals in Organic Aerosol Particles in Equilibrium with Nitric Acid Vapor" J. Phys. Chem. A, 2016, 120 (16), pp 2561–2568
- ↑ F. BUYTENDYK "A Study of the System Carbonic Acid, Carbon Dioxide and Water - Determination of the True Dissociation-constant of Carbonic Acid" Biochem J. 1927; 21(3): 576–584.
- ↑ Thiel and Strohecker (1914). Ber. deutsch. chem. Gem. 47, 945, 1061.