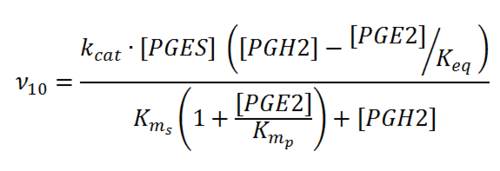Difference between revisions of "Transformation of PGH2 to PGE2"
(→Kmp) |
|||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
| − | PGE2 is | + | PGE2 is produced by the isomerisation of the PGH2 peroxide, into a ketone at C9 and an alcohol at C11 by PGES, yielding PGE2. This reaction is catalysed by isoforms of PGES, such as cPGES (also known as PGES-3), mPGES-1 and mPGES-2 <ref>Samuelsson, B. Morgenstern, R. Jakobsson, P. J. , ''Membrane prostaglandin E synthase-1: a novel therapeutic target'', Pharmacol Rev (2007), 59, 207-24.</ref>. cPGES is the cytosolic isoform of PGES which is constitutively expressed in the cytosol of various cells <ref>Tanioka, T. Nakatani, Y. Semmyo, N. Murakami, M. Kudo, I., ''Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis'', J Biol Chem (2000), 275, 32775-82.</ref>, whereas mPGES-1/-2 are Golgi membrane-associated proteins which only become cytosolic following spontaneous cleavage of a hydrophobic domain <ref>Murakami, M. Nakashima, K. Kamei, D. Masuda, S. Ishikawa, Y. Ishii, T. Ohmiya, Y. Watanabe, K. Kudo, I., ''Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2'', J Biol Chem (2003), 278, 37937-47.</ref><ref>Tanioka, T. Nakatani, Y. Semmyo, N. Murakami, M. Kudo, I., ''Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis'', J Biol Chem (2000), 275, 32775-82.</ref>. It has been suggested that a functional coupling of cPGES and COX-1 may occur in vitro as cPGES appears to convert only COX-1 derived PGH2 and not COX-2 derived PGH2 <ref>Tanioka, T. Nakatani, Y. Semmyo, N. Murakami, M. Kudo, I., ''Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis'', J Biol Chem (2000), 275, 32775-82.</ref>. Interestingly, the opposite has been observed for mPGES and COX-2 <ref>Murakami, M. Nakashima, K. Kamei, D. Masuda, S. Ishikawa, Y. Ishii, T. Ohmiya, Y. Watanabe, K. Kudo, I., ''Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2'', J Biol Chem (2003), 278, 37937-47.</ref>. |
| − | |||
| − | |||
| Line 25: | Line 23: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 34: | Line 33: | ||
pH: 8 | pH: 8 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |512 | ||
|<ref name="Pettersson2005"> [http://www.ncbi.nlm.nih.gov/pubmed/16399384 Pettersson P. , "Identification of beta-trace as prostaglandin D synthase.'' FASEB J. 2010 Dec;24(12):4668-77. doi: 10.1096/fj.10-164863. Epub 2010 Jul 28.]</ref> | |<ref name="Pettersson2005"> [http://www.ncbi.nlm.nih.gov/pubmed/16399384 Pettersson P. , "Identification of beta-trace as prostaglandin D synthase.'' FASEB J. 2010 Dec;24(12):4668-77. doi: 10.1096/fj.10-164863. Epub 2010 Jul 28.]</ref> | ||
|- | |- | ||
| Line 40: | Line 40: | ||
|Human | |Human | ||
|Wild Type Enzyme | |Wild Type Enzyme | ||
| + | |1024 | ||
|<ref name="Hamza2010"> [http://www.ncbi.nlm.nih.gov/pubmed/20369883 Hamza A. , "Understanding microscopic binding of human microsomal prostaglandin E synthase-1 (mPGES-1) trimer with substrate PGH2 and cofactor GSH: insights from computational alanine scanning and site-directed mutagenesis.'' J Phys Chem B. 2010 Apr 29;114(16):5605-16. doi: 10.1021/jp100668y.]</ref> | |<ref name="Hamza2010"> [http://www.ncbi.nlm.nih.gov/pubmed/20369883 Hamza A. , "Understanding microscopic binding of human microsomal prostaglandin E synthase-1 (mPGES-1) trimer with substrate PGH2 and cofactor GSH: insights from computational alanine scanning and site-directed mutagenesis.'' J Phys Chem B. 2010 Apr 29;114(16):5605-16. doi: 10.1021/jp100668y.]</ref> | ||
|- | |- | ||
| Line 51: | Line 52: | ||
Other: cPGES, casein kinase II and Hsp90 | Other: cPGES, casein kinase II and Hsp90 | ||
| + | |64 | ||
|<ref name="Kobayashi04"> [http://www.ncbi.nlm.nih.gov/pubmed/15040786 Kobayashi T. , "Regulation of cytosolic prostaglandin E synthase by phosphorylation.'' Biochem J. 2004 Jul 1;381(Pt 1):59-69.]</ref> | |<ref name="Kobayashi04"> [http://www.ncbi.nlm.nih.gov/pubmed/15040786 Kobayashi T. , "Regulation of cytosolic prostaglandin E synthase by phosphorylation.'' Biochem J. 2004 Jul 1;381(Pt 1):59-69.]</ref> | ||
|- | |- | ||
| Line 60: | Line 62: | ||
pH: Unknown | pH: Unknown | ||
Temperature: Unknown | Temperature: Unknown | ||
| + | |64 | ||
|<ref name="Kobayashi04"></ref> | |<ref name="Kobayashi04"></ref> | ||
|- | |- | ||
| Line 92: | Line 95: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 97: | Line 101: | ||
| per minute | | per minute | ||
| Human | | Human | ||
| − | + | |Expression Vector: E. Coli. | |
Enzyme: Microsomal Prostaglandin E Synthase | Enzyme: Microsomal Prostaglandin E Synthase | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 | Temperature: 37 | ||
| − | + | |1024 | |
| <ref name="Pettersson20005"> [www.ncbi.nlm.nih.gov/pubmed/16399384 Pettersson P., "Human microsomal prostaglandin E synthase 1: a member of the MAPEG protein superfamily.'' Methods Enzymol. 2005;401:147-61.]</ref> | | <ref name="Pettersson20005"> [www.ncbi.nlm.nih.gov/pubmed/16399384 Pettersson P., "Human microsomal prostaglandin E synthase 1: a member of the MAPEG protein superfamily.'' Methods Enzymol. 2005;401:147-61.]</ref> | ||
|- | |- | ||
| Line 107: | Line 111: | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the PGES kcat distribution | ||
! Mode (min-1) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ! Mode (min-1) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
|- | |- | ||
| Line 115: | Line 120: | ||
=== Enzyme concentration === | === Enzyme concentration === | ||
| + | |||
| + | To convert the enzyme concentration from ppm to mM, the following [[Common equations#Enzyme concentration (mM)|equation]] was used. | ||
| + | |||
{|class="wikitable sortable" | {|class="wikitable sortable" | ||
|+ style="text-align: left;" | Literature values | |+ style="text-align: left;" | Literature values | ||
| Line 122: | Line 130: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 131: | Line 140: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
|- | |- | ||
| Line 140: | Line 150: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 149: | Line 160: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
|- | |- | ||
| Line 158: | Line 170: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 167: | Line 180: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 173: | Line 187: | ||
{| class="wikitable" | {| class="wikitable" | ||
|+ style="text-align: left;" | Description of the PGES concentration distribution | |+ style="text-align: left;" | Description of the PGES concentration distribution | ||
| − | ! Mode !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | + | ! Mode (ppm) !! Mode (mM) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) |
|- | |- | ||
| − | | 7.49E+01 || 3.15E+00 || 5.03E+00 || 8.44E-01 | + | | 7.49E+01 ||4.15E-04 || 3.15E+00 || 5.03E+00 || 8.44E-01 |
|} | |} | ||
| Line 189: | Line 203: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 200: | Line 215: | ||
pH: 7.3 | pH: 7.3 | ||
ionic strength: 0.25 | ionic strength: 0.25 | ||
| + | |64 | ||
|<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=PROSTAGLANDIN-E-SYNTHASE-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | |<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=PROSTAGLANDIN-E-SYNTHASE-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | ||
|} | |} | ||
Latest revision as of 07:48, 21 August 2019
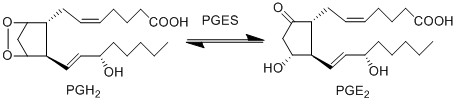
PGE2 is produced by the isomerisation of the PGH2 peroxide, into a ketone at C9 and an alcohol at C11 by PGES, yielding PGE2. This reaction is catalysed by isoforms of PGES, such as cPGES (also known as PGES-3), mPGES-1 and mPGES-2 [1]. cPGES is the cytosolic isoform of PGES which is constitutively expressed in the cytosol of various cells [2], whereas mPGES-1/-2 are Golgi membrane-associated proteins which only become cytosolic following spontaneous cleavage of a hydrophobic domain [3][4]. It has been suggested that a functional coupling of cPGES and COX-1 may occur in vitro as cPGES appears to convert only COX-1 derived PGH2 and not COX-2 derived PGH2 [5]. Interestingly, the opposite has been observed for mPGES and COX-2 [6].
Contents
Reaction
Chemical equation
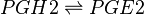
Rate equation
Parameters
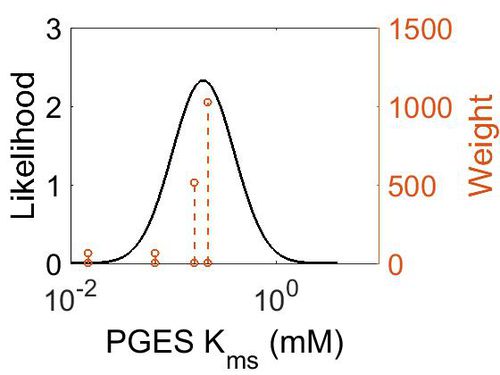
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 1.6E-01 ± 4.00E-03 | 
|
Human | Expression Vector: E. Coli
Enzyme: PGES pH: 8 Temperature: 37 |
512 | [7] |
| 2.15E-01 | 
|
Human | Wild Type Enzyme | 1024 | [8] |
| 1.49E-02 | 
|
Human | Expression Vector: E. Coli
Enzyme: PGES pH: Unknown Temperature: Unknown Other: cPGES, casein kinase II and Hsp90 |
64 | [9] |
| 6.66E-02 | 
|
Human | Expression Vector: E. Coli
Enzyme: PGES pH: Unknown Temperature: Unknown |
64 | [9] |
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 1.97E-01 | 1.73E+00 | -1.39E+00 | 4.91E-01 |
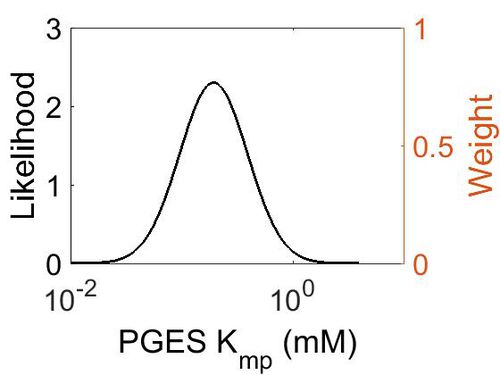
Kmp
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (mM) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 1.93E-01 | -1.15E+00 | 7.02E-01 (mM) |
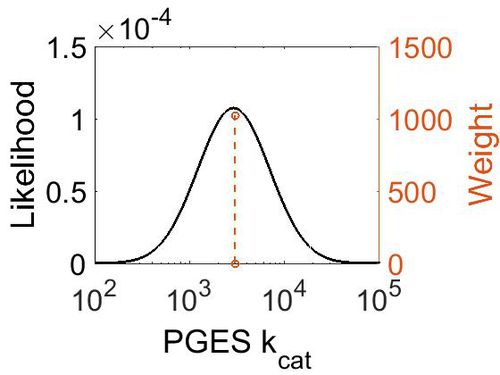
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 3000 ± 360 | per minute | Human | Expression Vector: E. Coli.
Enzyme: Microsomal Prostaglandin E Synthase pH: 7.5 Temperature: 37 |
1024 | [10] |
| Mode (min-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 2.98E+03 | 1.13E+00 | 8.01E+00 | 1.19E-01 |
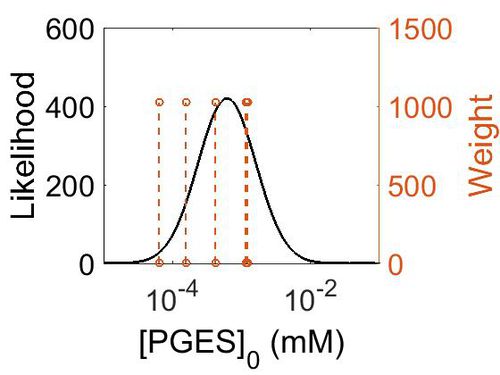
Enzyme concentration
To convert the enzyme concentration from ppm to mM, the following equation was used.
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 220 | 
|
Human | Expression Vector: Placenta
Enzyme: PGES pH: 7.5 Temperature: 37 °C |
1024 | [11] |
| 75.3 | 
|
Human | Expression Vector: Urinary Bladder
Enzyme: PGES pH: 7.5 Temperature: 37 °C |
1024 | [12] |
| 208 | 
|
Human | Expression Vector: Stomach
Enzyme: PGES pH: 7.5 Temperature: 37 °C |
1024 | [11] |
| 28.1 | 
|
Human | Expression Vector: Lung
Enzyme: PGES pH: 7.5 Temperature: 37 °C |
1024 | [12] |
| 11.6 | 
|
Human | Expression Vector: Colon
Enzyme: PGES pH: 7.5 Temperature: 37 °C |
1024 | [12] |
| Mode (ppm) | Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|---|
| 7.49E+01 | 4.15E-04 | 3.15E+00 | 5.03E+00 | 8.44E-01 |
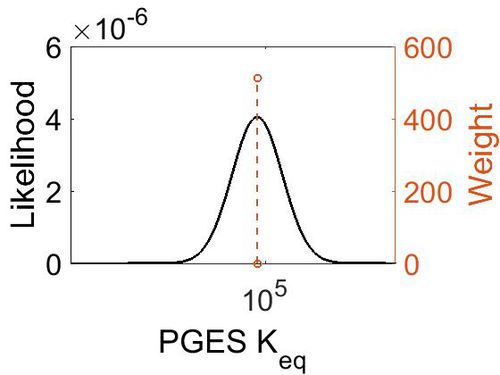
Keq
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 5.72 | kcal/mol | Not stated | Estimated
Enzyme: PGES Substrate: Arachidonate Product: PGE2 pH: 7.3 ionic strength: 0.25 |
64 | [13] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 7.46E+04 | 1.00E+01 | 1.20E+01 | 8.90E-01 |
References
- ↑ Samuelsson, B. Morgenstern, R. Jakobsson, P. J. , Membrane prostaglandin E synthase-1: a novel therapeutic target, Pharmacol Rev (2007), 59, 207-24.
- ↑ Tanioka, T. Nakatani, Y. Semmyo, N. Murakami, M. Kudo, I., Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis, J Biol Chem (2000), 275, 32775-82.
- ↑ Murakami, M. Nakashima, K. Kamei, D. Masuda, S. Ishikawa, Y. Ishii, T. Ohmiya, Y. Watanabe, K. Kudo, I., Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2, J Biol Chem (2003), 278, 37937-47.
- ↑ Tanioka, T. Nakatani, Y. Semmyo, N. Murakami, M. Kudo, I., Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis, J Biol Chem (2000), 275, 32775-82.
- ↑ Tanioka, T. Nakatani, Y. Semmyo, N. Murakami, M. Kudo, I., Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis, J Biol Chem (2000), 275, 32775-82.
- ↑ Murakami, M. Nakashima, K. Kamei, D. Masuda, S. Ishikawa, Y. Ishii, T. Ohmiya, Y. Watanabe, K. Kudo, I., Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2, J Biol Chem (2003), 278, 37937-47.
- ↑ Pettersson P. , "Identification of beta-trace as prostaglandin D synthase. FASEB J. 2010 Dec;24(12):4668-77. doi: 10.1096/fj.10-164863. Epub 2010 Jul 28.
- ↑ Hamza A. , "Understanding microscopic binding of human microsomal prostaglandin E synthase-1 (mPGES-1) trimer with substrate PGH2 and cofactor GSH: insights from computational alanine scanning and site-directed mutagenesis. J Phys Chem B. 2010 Apr 29;114(16):5605-16. doi: 10.1021/jp100668y.
- ↑ 9.0 9.1 Kobayashi T. , "Regulation of cytosolic prostaglandin E synthase by phosphorylation. Biochem J. 2004 Jul 1;381(Pt 1):59-69.
- ↑ [www.ncbi.nlm.nih.gov/pubmed/16399384 Pettersson P., "Human microsomal prostaglandin E synthase 1: a member of the MAPEG protein superfamily. Methods Enzymol. 2005;401:147-61.]
- ↑ 11.0 11.1 M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587
- ↑ 12.0 12.1 12.2 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471