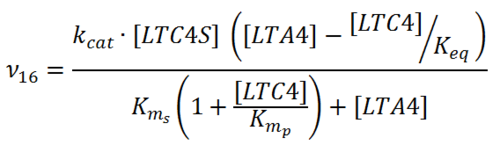Difference between revisions of "Transformation of LTA4 to LTC4"
(→Parameters) |
|||
| Line 24: | Line 24: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 33: | Line 34: | ||
pH:7.8 | pH:7.8 | ||
Temperature:20 °C | Temperature:20 °C | ||
| + | |256 | ||
|<ref name="Rinaldo2010"> [http://www.jbc.org/content/285/52/40771.full.pdf Rinaldo A. " Arginine 104 Is a Key Catalytic Residue in Leukotriene C4 | |<ref name="Rinaldo2010"> [http://www.jbc.org/content/285/52/40771.full.pdf Rinaldo A. " Arginine 104 Is a Key Catalytic Residue in Leukotriene C4 | ||
Synthase'' J Biochem 2010, 285, 40771-40776]</ref> | Synthase'' J Biochem 2010, 285, 40771-40776]</ref> | ||
| Line 43: | Line 45: | ||
pH: 7.8 | pH: 7.8 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Niegowski2013"> [http://www.jbc.org/content/early/2013/12/23/jbc.M113.534628 Niegowski D. " Crystal structures of Leukotriene C4 synthase in complex with product analogs, implications for the | |<ref name="Niegowski2013"> [http://www.jbc.org/content/early/2013/12/23/jbc.M113.534628 Niegowski D. " Crystal structures of Leukotriene C4 synthase in complex with product analogs, implications for the | ||
enzyme mechanism'' J. Biol. Chem. 289, 5199-5207 (2014)]</ref> | enzyme mechanism'' J. Biol. Chem. 289, 5199-5207 (2014)]</ref> | ||
| Line 75: | Line 78: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 84: | Line 88: | ||
pH:7.8 | pH:7.8 | ||
Temperature:20 °C | Temperature:20 °C | ||
| + | |256 | ||
|<ref name="Rinaldo2010"> [http://www.jbc.org/content/285/52/40771.full.pdf Rinaldo A. " Arginine 104 Is a Key Catalytic Residue in Leukotriene C4 | |<ref name="Rinaldo2010"> [http://www.jbc.org/content/285/52/40771.full.pdf Rinaldo A. " Arginine 104 Is a Key Catalytic Residue in Leukotriene C4 | ||
Synthase'' J Biochem 2010, 285, 40771-40776]</ref> | Synthase'' J Biochem 2010, 285, 40771-40776]</ref> | ||
| Line 94: | Line 99: | ||
pH: 7.8 | pH: 7.8 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Niegowski2013"> [http://www.jbc.org/content/289/8/5199.full Niegowski D. " Crystal structures of Leukotriene C4 synthase in complex with product analogs, implications for the | |<ref name="Niegowski2013"> [http://www.jbc.org/content/289/8/5199.full Niegowski D. " Crystal structures of Leukotriene C4 synthase in complex with product analogs, implications for the | ||
enzyme mechanism'' J. Biol. Chem. 289, 5199-5207 (2014)]</ref> | enzyme mechanism'' J. Biol. Chem. 289, 5199-5207 (2014)]</ref> | ||
| Line 116: | Line 122: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 125: | Line 132: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 134: | Line 142: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |2048 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 143: | Line 152: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 167: | Line 177: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 178: | Line 189: | ||
pH: 7.3 | pH: 7.3 | ||
ionic strength: 0.25 | ionic strength: 0.25 | ||
| + | |64 | ||
|<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=RXN66-490 Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | |<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=RXN66-490 Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | ||
|} | |} | ||
Revision as of 10:35, 22 May 2019
To generate LTC4, a supramolecular complex of 5-LOX, FLAP and leukotriene C4 synthase is formed on the nuclear membrane due to the increase of intracellular calcium \cite{Woods1993, Hammarberg2000, Radmark2015, Evans2008, Mandal2004, Mandal2008}
Contents
Reaction
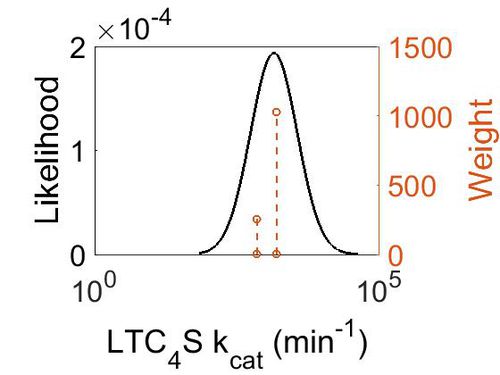
Chemical equation
Rate equation
Parameters
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 0.3 �± 0.06 | mM | Human | Expression Vector: E. Coli.
Enzyme: Wild Type hLTC4S pH:7.8 Temperature:20 °C |
256 | [1] |
| 3.00E-02 ± 1.00E-02 | mM | Human | Expression Vector: E Coli
Enzyme: Wild type LTC4S pH: 7.8 Temperature: 37 °C |
1024 | [2] |
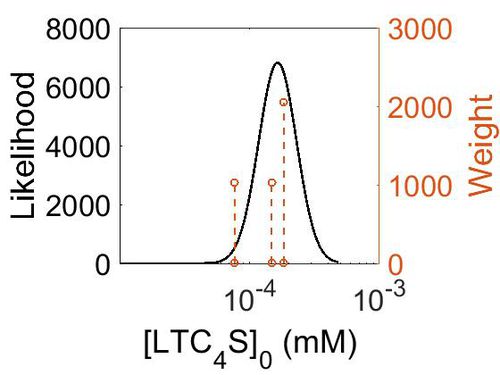
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 3.15E-02 | 7.18E+00 | -2.83E+00 | 7.90E-01 |
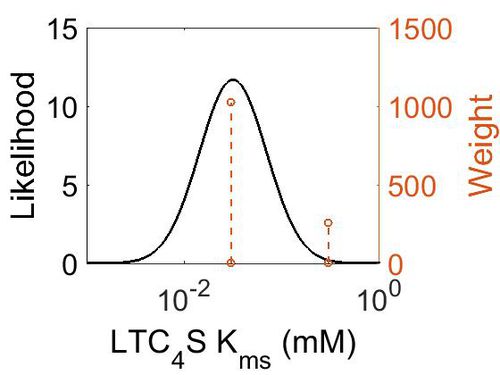
Kmp
| Mode (mM) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 3.18E-02 | -2.82E+00 | 7.92E-01 |
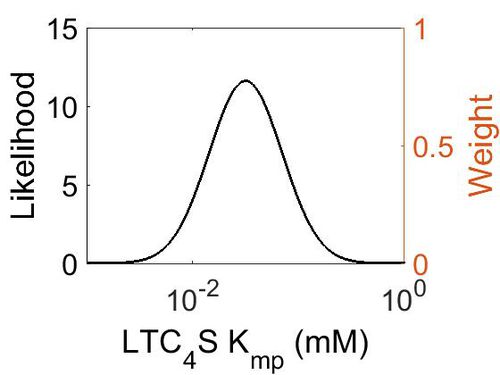
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 702 | per minute | Human | Expression Vector: E. Coli.
Enzyme: Wild Type hLTC4S pH:7.8 Temperature:20 °C |
256 | [1] |
| 1560 ± 240 | per minute | Human | Expression Vector: E Coli
Enzyme: Wild type LTC4S pH: 7.8 Temperature: 37 °C |
1024 | [2] |
| Mode (min-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 1.47E+03 | 1.42E+00 | 7.40E+00 | 3.29E-01 |
Enzyme concentration
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 26.8 | 
|
Human | Expression Vector: Lung
Enzyme: LTC4S pH: 7.5 Temperature: 37 °C |
1024 | [3] |
| 33.0 | 
|
Human | Expression Vector: Esophagus
Enzyme: LTC4S pH: 7.5 Temperature: 37 °C |
2048 | [3] |
| 13.9 | 
|
Human | Expression Vector: Adrenal Gland
Enzyme: LTC4S pH: 7.5 Temperature: 37 °C |
1024 | [3] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 2.90E+01 | 1.44E+00 | 3.49E+00 | 3.46E-01 |
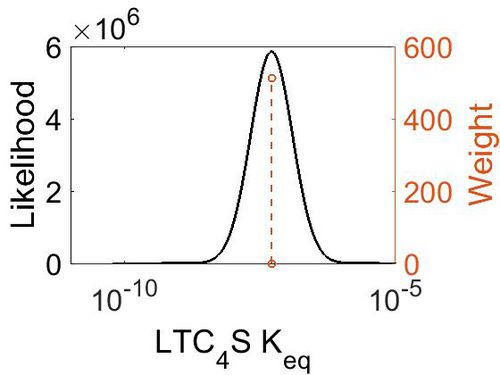
Keq
| Gibbs Free Energy Change | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 9.934128 | kcal/mol | Not stated | Estimated
Enzyme: LTC4S Substrate: LTA4 Product: LTC4 pH: 7.3 ionic strength: 0.25 |
64 | [4] |
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 5.13E-08 | 1.00E+01 | -1.60E+01 | 8.90E-01 |
Related Reactions
References
- ↑ 1.0 1.1 [http://www.jbc.org/content/285/52/40771.full.pdf Rinaldo A. " Arginine 104 Is a Key Catalytic Residue in Leukotriene C4 Synthase J Biochem 2010, 285, 40771-40776]
- ↑ 2.0 2.1 [http://www.jbc.org/content/early/2013/12/23/jbc.M113.534628 Niegowski D. " Crystal structures of Leukotriene C4 synthase in complex with product analogs, implications for the
enzyme mechanism J. Biol. Chem. 289, 5199-5207 (2014)] Cite error: Invalid
<ref>tag; name "Niegowski2013" defined multiple times with different content - ↑ 3.0 3.1 3.2 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471