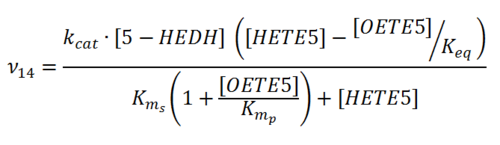Transformation of 5-HETE to 5-OXO-ETE
5-HETE is oxidised by 5-hydroxyeicosanoid dehydrogenase (5-HEDH) to 5-oxo-ETE [1]. 5-HEDH is a microsomal enzyme which catalyses the conversion of the C5 alcohol to a ketone by transferring the hydrogen cation to NADP+ via a ping-pong mechanism [2][3].
Contents
Reaction
Chemical equation
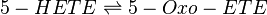
Rate equation
Parameters
Note that there was limited availability of kinetic data for this specific enzyme, therefore parameters for general alcohol dehydrogenase (ADH) was used when non were available.
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 0.0006 | mM | Unkown | Follows a ping-pong mechanism by binding with NADP+, transforming it to NADPH and releasing it before binding 5S-HETE. | 64 | [4] |
| 0.00067 | mM | Human Cell lines | Follows a ping-pong mechanism by binding with NADP+ (Km 139 nM), transforming it to NADPH (inhibited by NADPH (Ki 224 nM)) and releasing it before binding 5S-HETE.
Method: In vitro Organism: Human Expression vector: U937 and HL-60 cell lines Enzyme:5-HEDH pH: 7.4 Temperature: 37 ◦C |
2048 | [5] |
| 0.000516 ± 0.00019 | mM | Human Cell lines |
Method: In vitro Organism: Human Expression vector: U937 Enzyme:5-HEDH pH: 7.4 Temperature: 37 ◦C |
2048 | [6] |
| Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 6.34E-04 | 1.63E+01 | -6.31E+00 | 1.03E+00 |
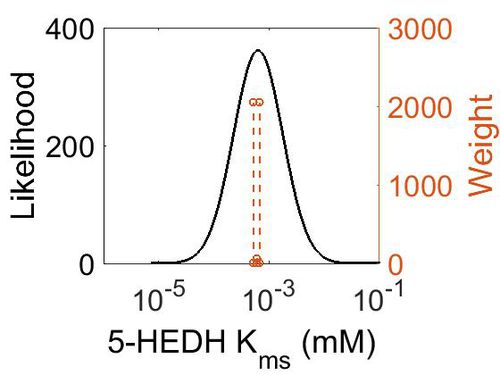
Kmp
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (mM) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 1.14E+04 | 1.09E+01 | 1.25E+00 |
Vmax
| Value | Units | Species | Notes | Reference |
|---|---|---|---|---|
| 0.54 ± 0.30 | pmol/ mi mg | Human Cell lines - Neutrophils |
Method: In vitro Organism: Human Expression vector: U937 and HL-60 cell lines Enzyme:5-HEDH pH: 7.4 Temperature: 37 ◦C |
[5] |
| 0.40 ± 0.12 | pmol/ mi mg | Human Cell lines - U937 |
Method: In vitro Organism: Human Expression vector: U937 and HL-60 cell lines Enzyme:5-HEDH pH: 7.4 Temperature: 37 ◦C |
[5] |
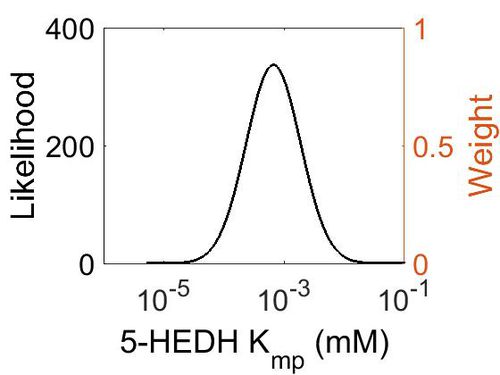
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 11640 ± 840 | min-1 | Yokenella | Method: In vitro
Organism: Human Expression vector: E Coli Enzyme: pH: 6.5 Temperature: 65 ◦C Substrate: NADPH |
128 | [7] |
| Mode (min-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 1.15E+04 | 4.02E+01 | 1.09E+01 | 1.25E+00 |
Enzyme concentration (Using ADH5 abundance data)
To convert the enzyme concentration from ppm to mM, the following equation was used.
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 490 | ppm | Human | Expression Vector: Skin
Enzyme: ADH5 pH: 7.5 Temperature: 37 °C |
2048 | [8] |
| 625 | ppm | Human | Expression Vector: Oral Cavity
Enzyme: ADH5 pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| 728 | ppm | Human | Expression Vector: Esophagus
Enzyme: ADH5 pH: 7.5 Temperature: 37 °C |
1024 | [9] |
| 11.5 | ppm | Human | Expression Vector: Skin
Enzyme: ADH5 pH: Unknown Temperature: Unknown |
2048 | [9] |
| Mode (ppm) | Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|---|
| 4.88E+02 | 2.70E-03 | 6.37E+00 | 7.49E+00 | 1.14E+00 |
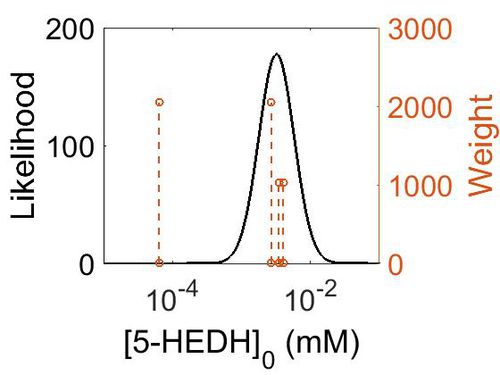
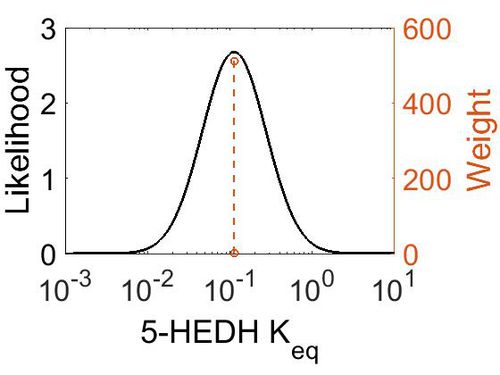
Keq
| Gibbs Free energy | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 1.29 | kcal/mol | Arabidopsis thaliana col | Same reaction, no prediction available for 5-HEDH on Metacyc
Estimated Enzyme: ω-hydroxy fatty acid ω-alcohol dehydrogenase Substrate: 18-hydroxyoleate Product: 18-oxo-oleate pH: 7.3 ionic strength: 0.25 |
64 | [10] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 1.12E-01 | 1.00E+01 | -1.39E+00 | 8.91E-01 |
Related Reactions
References
- ↑ Powell, W. S. Gravelle, F. Gravel, S. , Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes, J Biol Chem (1992), 267, 19233-41.
- ↑ Powell, W. S. Gravelle, F. Gravel, S. , Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes, J Biol Chem (1992), 267, 19233-41.
- ↑ Erlemann, K. Cossette, C. Grant, G. Lee, G. Patel, P. Rokach, J. Powell, W., Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells, Biochem J (2007), 403, 157-165
- ↑ Lipoxygenases in Inflammation - edited by Dieter Steinhilber
- ↑ 5.0 5.1 5.2 [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1828885/pdf/bj4030157.pdf Karl-Rudolf ERLEMANN, Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells, Biochem. J. (2007) 403, 157–165]
- ↑ [http://jpet.aspetjournals.org/content/jpet/329/1/335.full.pdf Karl-Pranav Patel, Selectivity of 5-Hydroxyeicosanoid Dehydrogenase and Its Inhibition by 5-Hydroxy-Long-Chain Fatty Acids, Journal of Pharmacology and Experimental Therapeutics April 2009, 329 (1) 335-341; ]
- ↑ Cong Wei, The Role of 15-Lipoxygenase-1- and Cyclooxygenase-2-Derived Lipid Mediators in Endothelial Cell Proliferation, Thesis (2010)
- ↑ 8.0 8.1 [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587]
- ↑ 9.0 9.1 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471