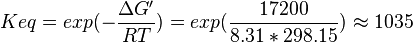Phosphofructokinase type 1
The enzyme Phosphofructokinase Type-1 uses another ATP molecule to transfer a phosphate group to Fru6P to form fructose 1, 6-bisphosphate. PFK-1 is an allosteric enzyme showing cooperative behaviour with Fru6P and hyperbolic kinetics with ATP.
Contents
Chemical equation
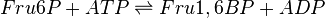
Rate equation
The concerted transition model of Monod, Wyman and Changeux (MWC model) is used as a rate equation for this tetrameric enzyme for considering exclusive ligand binding (F6P, activators and inhibitors) together with mixed type activation, (Fru2,6BP or AMP or Pi) [1].
![v = Vm \left(\frac{\frac{[ATP]}{Km_{ATP}}}{1 + \frac{[ATP]}{Km_{ATP}} }\right ) \left ( \frac{ 1 + \frac{\beta[Fru2,6BP]}{ \alpha Ka_{Fru2,6BP} } }{ 1 + \frac{[Fru2,6BP]}{ \alpha Ka_{Fru2,6BP} } } \right )
\left( \frac{\frac{[Fru6P]\left(1+\frac{[Fru2,6BP]}{[\alpha Ka_{Fru2,6BP}]}\right)}{Km_{Fru6P}\left(1 + \frac{[Fru2,6BP]}{Ka_{Fru2,6BP}}\right)} \left[1 + \frac{[Fru6P]\left(1+\frac{[Fru2,6BP]}{\alpha Ka_{Fru2,6BP}}\right)}{Km_{Fru6P}\left(1 + \frac{[Fru2,6BP]}{Ka_{Fru2,6BP}}\right)} \right]^3}
{ \frac{L\left( 1 + \frac{[CIT]}{Ki_{CIT}}\right)^4\left(1 + \frac{[ATP]}{Ki_{ATP}}\right)^4}{\left(1+\frac{[Fru2,6BP]}{Ka_{Fru2,6BP}}\right)^4} + \left[1 + \frac{Fru6P\left(1+\frac{Fru2,6BP}{\alpha Ka_{Fru2,6BP}}\right)}{Km_{Fru6P}\left(1 + \frac{[Fru2,6BP]}{Ka_{Fru2,6BP}}\right)} \right]^4 } - \left( \frac{\frac{[ADP][Fru1,6BP]}{K_{ADP}K_{Fru1,6BP}K_{eq}}}{\frac{[ADP]}{K_{ADP}} + \frac{[Fru1,6BP]}{K_{Fru1,6BP}} + \frac{[ADP][Fru1,6BP]}{K_{ADP}K_{Fru1,6BP}} + 1 } \right) \right)](/wiki/images/math/9/b/0/9b0e838e3bb1a1ed51a5745253a99625.png)
Parameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|
|
0.031 [1] | 
|
HeLa cell line | Moreno-Sánchez, Marín-Hernández, Encalada & Saavedra, unpublished results |
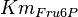
|
1.0 [1] | mM | ||
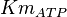
|
0.021[1] | mM | ||
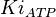
|
20[1] | mM | ||
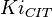
|
6.8[1] | mM | ||
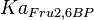
|
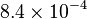 [1] [1]
|
mM | ||
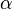
|
0.32[1] | Dimensionless | ||
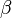
|
0.98[1] | Dimensionless | ||
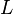
|
4.1[1] | Dimensionless | ||
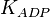
|
5[1] | mM | ||
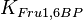
|
5[1] | mM | ||

|
247[1] | mM | Recalculated from the 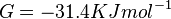
|
Parameters with uncertainty
- The
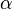 and
and 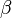 in the rate equation represents the factors by which the ligand affinity and catalytic capacity are modified in the presence of an allosteric activatory [2]. As
in the rate equation represents the factors by which the ligand affinity and catalytic capacity are modified in the presence of an allosteric activatory [2]. As 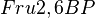 is the only activator in our model we considered the
is the only activator in our model we considered the 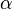 and
and 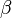 value of
value of 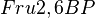 ,
, 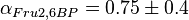 and
and 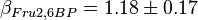 . These two values are measured in the presence of 140
. These two values are measured in the presence of 140 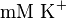 medium [2].
medium [2].
- The Vm value is reported as
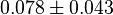 U \times (mg protein)^{-1} [2]. HeLa cells were harvested at a concentration of 65 mg protein/ml. Converting Vm to mM/min becomes
U \times (mg protein)^{-1} [2]. HeLa cells were harvested at a concentration of 65 mg protein/ml. Converting Vm to mM/min becomes 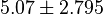 again in the presence of 140
again in the presence of 140 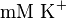 .
.
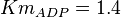 for Thermotoga maritima is being reported in Hansen, T., M. Musfeldt et. al. [3] and
for Thermotoga maritima is being reported in Hansen, T., M. Musfeldt et. al. [3] and 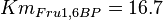 for Desulfurococcus amylolyticus is reported in Hansen T, Schönheit P. et. al.[4]. The mean and std. dev. is calculated as
for Desulfurococcus amylolyticus is reported in Hansen T, Schönheit P. et. al.[4]. The mean and std. dev. is calculated as 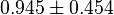
- Similarly for
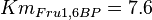 in Thermotoga maritima is being reported in Hansen, T., M. Musfeldt et. al. [3] and
in Thermotoga maritima is being reported in Hansen, T., M. Musfeldt et. al. [3] and 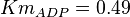 in Desulfurococcus amylolyticus is reported in Hansen T, Schönheit P. et. al.[4]. The mean and std. dev. is calculated as
in Desulfurococcus amylolyticus is reported in Hansen T, Schönheit P. et. al.[4]. The mean and std. dev. is calculated as 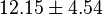
- Four Keq values have been reported in the SilicoTrypWiki (Wikipedia for insilico modelling of Trypanosome) for Phosphofructokinase: 308.4, 254, 1035, 800[5]. As the
 value does not depend on the organism, the mean and the standard deviation can be calculated from these 4 values collected for Trypanosome.[6]. The mean and std. dev. of this value is
value does not depend on the organism, the mean and the standard deviation can be calculated from these 4 values collected for Trypanosome.[6]. The mean and std. dev. of this value is 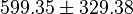
| Parameter | Value | Units | Organism |
|---|---|---|---|
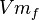
|
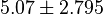
|
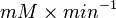
|
HeLa Cell line |
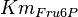
|
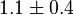
|
mM | HeLa Cell line |
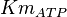
|
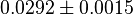 [2] [2]
|
mM | HeLa Cell line |
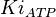
|
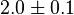 [7] [7]
|
mM | Human muscle |
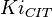
|
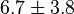
|
mM | HeLa Cell line |
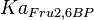
|
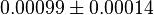
|
mM | HeLa Cell line |
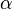
|
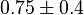
|
Dimensionless | HeLa Cell line |
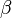
|
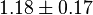
|
Dimensionless | HeLa Cell line |
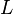
|
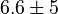
|
Dimensionless | HeLa Cell line |
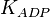
|
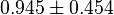
|
mM | Thermotoga maritima & Desulfurococcus amylolyticus |
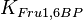
|
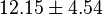
|
mM | Thermotoga maritima & Desulfurococcus amylolyticus |

|
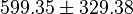
|
Trypanosome |
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 308.4 | pH=7, T=25°C | Lehninger, (2008)[8] p 553:
|
| 254 | pH=7, T=25°C | Lehninger, (1975)[9] p 396. |
| 1035 | pH=7, T=25°C | Voet et al.[10] from Newshole et al. (1973) [11]p 97:
|
| 800 | T=298.15 K; pH=7.0; Method: calorimetry; Buffer: Tris (0.1 mol dm-3) + HCl. | NIST database "Thermodynamics of Enzyme-Catalyzed Reactions" entry [75BOH/SCH_551] from Bvhme et al. (1975)[12] |
- The Average from these values are
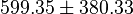
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011). Modeling cancer glycolysis. Biochim Biophys Acta, 1807:755–767 (doi)
- ↑ 2.0 2.1 2.2 2.3 R. Moreno-Sánchez, A. Marín-Hernández, J.C. Gallardo-Pérez, H. Quezada, R. Encalada, S. Rodríguez-Enríquez et al. (2012), Phosphofructokinase type 1 kinetics, isoform expression, and gene polymorphisms in cancer cells, J Cell Biochem, 113, pp. 1692–1703
- ↑ 3.0 3.1 Hansen, T., M. Musfeldt, and P. Schonheit (2002), ATP-dependent 6-phosphofructokinase from the hyperthermophilic bacterium Thermotoga maritima: characterization of an extremely thermophilic, allosterically regulated enzyme. Arch. Microbiol. 177:401-409
- ↑ 4.0 4.1 Hansen T, Schönheit P. (2003),Purification and Characterization of the MQH2:NO Oxidoreductase from the Hyperthermophilic Archaeon Pyrobaculum aerophilum, J Biol Chem, 278 (38), 35861-35868
- ↑ [Silicotryp]
- ↑ F. Achcar, E.J. Kerkhoven, B.M. Bakker, M.P. Barrett, R. Breitling (2012), Dynamic modelling under uncertainty: the case of Trypanosoma brucei energy metabolism, PLoS Comput Biol, 8, p. e1002352
- ↑ Brueser, A.; Kirchberger, J.; Kloos, M.; Straeter, N.; Schoeneberg, T. (2012), Functional linkage of adenine nucleotide binding sites in mammalian muscle 6-phosphofructokinase, J. Biol. Chem. 287, 17546-17553 (2012)
- ↑ David L. Nelson, Michael M. Cox (2008), Lehninger Principles of Biochemistry (5th edn), W. H. Freeman and Company
- ↑ Lehninger, A.L. (1975) Biochemistry (2nd edn), Worth
- ↑ Voet, D., Voet., J.G. and Pratt, C. W. (1999) Fundamentals of biochemistry, Wiley
- ↑ Newshole, E.A. and Stuart, C. (1973) Regulation in Metabolism, Wiley
- ↑ Bvhme, H.-J., Schellenberger, W. and Hofmann, E. (1975) Acta Biol. Med. Germ. 34(1):15-20. (pmid: 241184)
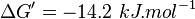 ,
, 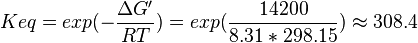
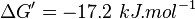 ,
,