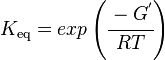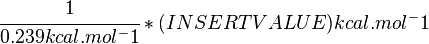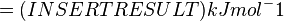Limonene-3-hydroxylase (L3H)
You can go back to main page of the kinetic model here.
Contents
Reaction catalysed
Enzyme and Metabolite Background Information
Long metabolite names are abbreviated in the model for clarity and standard identification purposes.
| Metabolite | Abbreviation | Chemical Formula | Molar mass (g/mol) | ChEBI | ChEMBL | PubChem | BRENDA | PlantCyc |
|---|---|---|---|---|---|---|---|---|
| limonene-3-hydroxylase | L3H | 56.1 kD | 1.14.13.47 | MONOMER-6761 | ||||
| (-)-4S-limonene | limonene | C10H16 | 136.24 | 15384 | 449062 | 22311 or 439250 | ||
| (-)-trans-isopiperitenol | isopiperitenol | C10H16O | 152.23344 | 15406 | 439410 | |||
| NADPH | C21H30N7O17P3 | 745.42116 | 16474 | |||||
| NADP+ | C21H29N7O17P3 | 744.41322 | 18009 | |||||
| Dioxygen | O2 | O2 | 31.99880 | 15379 | ||||
| water | H2O | H2O | 18.01530 | 15377 |
Equation Rate
Limonene-hydroxylase (L3H) is modelled using the reversible Michaelis-Menten equation.
| Parameter | Description | Reference |
|---|---|---|
| VL3H | Reaction rate for Limonene-3-hydroxylase | ref |
| Vmaxforward | Maximum reaction rate towards the production of (-)-trans-isopiperitenol | ref |
| Kmlimonene | Michaelis-Menten constant for Limonene | ref |
| Kmisopiperitenol | Michaelis-Menten constant for (-)-trans-isopiperitenol | ref |
| KmNADPH | Michaelis-Menten constant for NADPH | ref |
| KmNADP | Michaelis-Menten constant for NADP+ | ref |
| Keq | Equilibrium constant | ref |
| [limonene] | Limonene concentration | ref |
| [isopiperitenol] | (-)-trans-isopiperitenol concentration | ref |
| [NADPH] | NADPH concentration | ref |
| [NADP] | NADP+ concentration | ref |
Strategies for estimating the kinetic parameter values
Calculating the Equilibrium Constant
The equilibrium constant can be calculated using the Van't Hoff Isotherm equation:
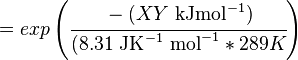
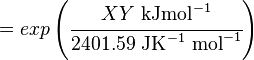
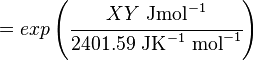
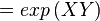
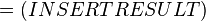
where;
| Keq | Equilibrium constant |
| -ΔG° | Gibbs free energy change. For (INSERT ENZYME) it is (INSERT VALUE) kJmol-1 |
| R | Gas constant with a value of 8.31 JK-1mol-1 |
| T | Temperature which is always expressed in kelvin |
Standard Gibbs Free energy
Standard Gibbs Free energy for (INSERT ENZYME) from MetaCyc (EC 4.2.3.16) is (INSERT VALUE) kcal/mol [1].
SI derived unit for Gibbs free energy is Joules per mol (J mol-1). 1 kJ·mol−1 is equal to 0.239 kcal·mol−1.
Therefore, the Gibbs free energy for (INSERT ENZYME) in kJ mol-1 is:
Extracting Information from (INSERT SUBSTRATE/PRODUCT) Production Rates
| Amount produced (mg/L) | Time (H) | Organism | Description | Reaction Flux (µM/s) |
|---|---|---|---|---|
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
| X | X | Y | Z | Z |
Published Kinetic Parameter Values
| Km (mM) | Vmax | Kcat (s-1) | Kcat/Km | Organism | Description |
|---|---|---|---|---|---|
| 0.00125 | - | - | - | Z | A -> B |
| 0.0018 | - | - | - | Z | A -> B |
| Y | Y | Y | Y | Z | A -> B |
| Y | Y | Y | Y | Z | A -> B |
| Y | Y | - | - | Z | A -> B |
| Y | - | Y | - | Z | GPP -> B |
| Y | - | Y | - | Z | GPP -> B |
| x | - | y | - | Z. | A -> B |
Detailed description of kinetic values obtained from literature
A more detailed description of the values listed above can be found here .
Simulations
References
- ↑ Latendresse M. (2013). "Computing Gibbs Free Energy of Compounds and Reactions in MetaCyc."
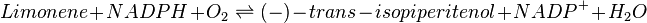
![V_\mathrm{L3H} = Vmax_\mathrm{forward} * \cfrac {\left ( \cfrac{[limonene]}{Km_\mathrm{limonene}} * \cfrac {[NADPH]}{Km_\mathrm{NADPH}} \right ) * \left ( 1 - \cfrac {[isopiperitenol]*[NADP]}{[limonene]*[NADPH]*K_\mathrm{eq}} \right )}
{ \left (1 + \cfrac {[NADPH]}{Km_\mathrm{NADPH}} + \cfrac {[NADP]}{Km_\mathrm{NADP}} \right ) + \left ( 1+ \cfrac {[limonene]}{Km_\mathrm{limonene}} + \cfrac {[isopiperitenol]}{Km_\mathrm{isopiperitenol}} \right ) }](/wiki/images/math/6/3/7/6372d5716522f3a6100b47a63f36e873.png)