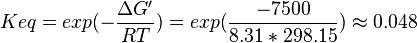Difference between revisions of "Triosephosphate isomerase"
(→Parameters with uncertainty) |
(→Parameters with uncertainty) |
||
| Line 76: | Line 76: | ||
|<math>K_{eq}(reverse) = 20.9, and so K_{eq}(forward) = \frac{1}{20.9} = 0.047 </math> | |<math>K_{eq}(reverse) = 20.9, and so K_{eq}(forward) = \frac{1}{20.9} = 0.047 </math> | ||
|} | |} | ||
| + | |||
| + | ===Equilibrium constant=== | ||
| + | {| border="1" cellpadding="2" | ||
| + | ! Equilibrium constant | ||
| + | ! Conditions | ||
| + | ! Source | ||
| + | |- | ||
| + | | 0.045 | ||
| + | | pH=8, T=25°C | ||
| + | | Bergmeyer ''Methods of enzymatic analysis'' page 515<ref name="bermeyer74">Bergmeyer H.U. (1974) ''Methods of enzymatic analysis'', Publisher: Verlag Chemie (vol 1)</ref> | ||
| + | |- | ||
| + | | 0.041 | ||
| + | | pH=7, T=25°C | ||
| + | | Voet et al.<ref name="voet">Voet, D., Voet., J.G. and Pratt, C. W. (1999) Fundamentals of biochemistry, Wiley</ref> from Newshole et al. (1973) <ref name="newshole73">Newshole, E.A. and Stuart, C. (1973) Regulation in Metabolism, Wiley</ref>p 97:<br/> | ||
| + | <math>\Delta G' = 7.9\ kJ.mol^{-1}</math>, <math>Keq = exp(-\frac{\Delta G'}{RT}) = exp(\frac{-7900}{8.31*298.15}) \approx 0.041</math> | ||
| + | |- | ||
| + | | 0.048 | ||
| + | | pH=7, T=25°C | ||
| + | | Lehninger, (1975)<ref name="lehninger75">Lehninger, A.L. (1975) Biochemistry (2nd edn), Worth</ref> p 408:<br/> | ||
| + | <math>\Delta G' = 7.5\ kJ.mol^{-1}</math>, <math>Keq = exp(-\frac{\Delta G'}{RT}) = exp(\frac{-7500}{8.31*298.15}) \approx 0.048</math> | ||
| + | |- | ||
| + | | 0.0475 | ||
| + | | pH=7, T=25°C | ||
| + | | Lehninger, (1975)<ref name="lehninger75">Lehninger, A.L. (1975) Biochemistry (2nd edn), Worth</ref> p 396. | ||
| + | |} | ||
| + | |||
| + | *Taking average of all those values give <math>0.0457 \pm 0.002863</math> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 14:07, 12 August 2014
This enzyme rapidly inter-converts the molecules Dihydroxyacetone phosphate (DHAP) and Glyceraldehyde 3-phosphate (Gly3P). Gly3P is removed as soon as it is formed to be used in the next step of glycolysis.
Contents
Chemical equation
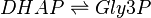
Rate equation
Reversible Michaelis-Menten is used [1]
![v = \frac{ V_{mf}\frac{[DHAP]}{K_{DHAP}} - V_{mr}\frac{[Gly3P]}{K_{Gly3P}} }{1 + \frac{[DHAP]}{K_{DHAP}} + \frac{[Gly3P]}{K_{Gly3P}} }](/wiki/images/math/c/4/3/c43ea2245f63ddca8689602949bdf9f4.png)
Modified rate law considering thermodynamic constant is
![v = \frac{ V_{mf}\frac{[DHAP]}{K_{DHAP}}\left(1 - \frac{[Gly3P]}{K_{eq}[DHAP]} \right)}{1 + \frac{[DHAP]}{K_{DHAP}} + \frac{[Gly3P]}{K_{Gly3P}} }](/wiki/images/math/0/a/2/0a21e55dde0aa7699f6d0a6b766641a7.png)
Paramters
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
5 [1] | 
|
Hela cell line | |

|
42[2] | 
| ||
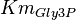
|
0.51[1] | mM | ||
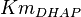
|
1.6[1] | mM |
Parameters with uncertainty
- The activity is measured in Activity in the reverse reaction in Hernandez (2006) et. al.
 is sampled based on Haldane equation
is sampled based on Haldane equation 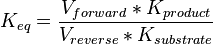 using the value Failed to parse (Cannot store math image on filesystem.): K_{eq} = 20.9 \pm 3.1
,
using the value Failed to parse (Cannot store math image on filesystem.): K_{eq} = 20.9 \pm 3.1
, 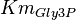 and
and 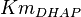 .
.
Alternative-1 the reported fixed point value can be considered with the standard deviation calculated based on the same ratio of  which is
which is 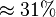 . This gives the value Failed to parse (Cannot store math image on filesystem.): V_{mf}=5 \pm 1.55
. This gives the value Failed to parse (Cannot store math image on filesystem.): V_{mf}=5 \pm 1.55
Alternative-2 Calculating  from
from  based on Haldane equation which gives the value of 2.911 and with the same percent of erro Std. Dev. is 0.90.
based on Haldane equation which gives the value of 2.911 and with the same percent of erro Std. Dev. is 0.90.
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
Sampled based on the Haldane equation. Alternative: Failed to parse (Cannot store math image on filesystem.): 5 \pm 1.55 or 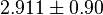
|
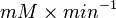
|
||

|
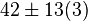 [2] [2]
|
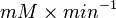
| ||
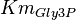
|
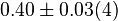 [3] [3]
|
mM | Human liver | |
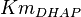
|
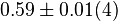 [3] [3]
|
mM | Human liver | |

|
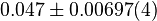 [3] [3]
|
mM | 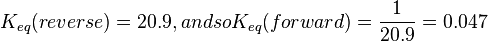
|
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 0.045 | pH=8, T=25°C | Bergmeyer Methods of enzymatic analysis page 515[4] |
| 0.041 | pH=7, T=25°C | Voet et al.[5] from Newshole et al. (1973) [6]p 97:
|
| 0.048 | pH=7, T=25°C | Lehninger, (1975)[7] p 408:
|
| 0.0475 | pH=7, T=25°C | Lehninger, (1975)[7] p 396. |
- Taking average of all those values give
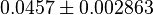
References
- ↑ 1.0 1.1 1.2 1.3 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi) Cite error: Invalid
<ref>tag; name "Hernandez2011" defined multiple times with different content - ↑ 2.0 2.1 Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)
- ↑ 3.0 3.1 3.2 Snyder, R.; Lee, E.W. (1975), Triosephosphate isomerase from human and horse liver,Methods Enzymol. 41B, 430-434
- ↑ Bergmeyer H.U. (1974) Methods of enzymatic analysis, Publisher: Verlag Chemie (vol 1)
- ↑ Voet, D., Voet., J.G. and Pratt, C. W. (1999) Fundamentals of biochemistry, Wiley
- ↑ Newshole, E.A. and Stuart, C. (1973) Regulation in Metabolism, Wiley
- ↑ 7.0 7.1 Lehninger, A.L. (1975) Biochemistry (2nd edn), Worth
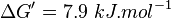 ,
, 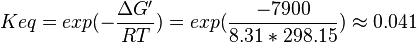
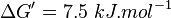 ,
,