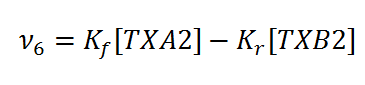Difference between revisions of "Transformation of TXA2 to TXB2"
(→Parameters) |
(→Parameters) |
||
| Line 24: | Line 24: | ||
! Conditions | ! Conditions | ||
! Substrate | ! Substrate | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 31: | Line 32: | ||
Temperature: 25°C | Temperature: 25°C | ||
|3 | |3 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 38: | Line 40: | ||
Temperature: 25°C | Temperature: 25°C | ||
|3 | |3 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 45: | Line 48: | ||
Temperature: 25°C | Temperature: 25°C | ||
|4 | |4 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 52: | Line 56: | ||
Temperature: 25°C | Temperature: 25°C | ||
|4 | |4 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 59: | Line 64: | ||
Temperature: 25°C | Temperature: 25°C | ||
|5 | |5 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 66: | Line 72: | ||
Temperature: 25°C | Temperature: 25°C | ||
|6 | |6 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 73: | Line 80: | ||
Temperature: 25°C | Temperature: 25°C | ||
|7 | |7 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 80: | Line 88: | ||
Temperature: 25°C | Temperature: 25°C | ||
|8 | |8 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 87: | Line 96: | ||
Temperature: 25°C | Temperature: 25°C | ||
|8 | |8 | ||
| + | |32 | ||
|<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | |<ref name="Ross1982”>[http://pubs.acs.org/doi/pdf/10.1021/ja00370a035 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665]</ref> | ||
|- | |- | ||
| Line 93: | Line 103: | ||
| 25°C, in imidazole buffer and also in phosphate buffers, | | 25°C, in imidazole buffer and also in phosphate buffers, | ||
| CO2 to H2CO3 | | CO2 to H2CO3 | ||
| + | |32 | ||
| <ref name="Gibbons1963”>[http://www.jbc.org/content/238/10/3502.full.pdf B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7]</ref> | | <ref name="Gibbons1963”>[http://www.jbc.org/content/238/10/3502.full.pdf B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7]</ref> | ||
|- | |- | ||
| Line 126: | Line 137: | ||
! Conditions | ! Conditions | ||
! Substrate | ! Substrate | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 132: | Line 144: | ||
|Temperature: 35°C | |Temperature: 35°C | ||
Vector:Mosquito | Vector:Mosquito | ||
| − | |||
Note: "In solution, it undergoes rapid hydrolysis to form TXB2, a stable but physiologically inactive compound." - therefore they used stable analogues. | Note: "In solution, it undergoes rapid hydrolysis to form TXB2, a stable but physiologically inactive compound." - therefore they used stable analogues. | ||
|carbocyclic TXA2 (analogue of TXA2) | |carbocyclic TXA2 (analogue of TXA2) | ||
| + | |24 | ||
|<ref name="Alvarenga2010”>[http://journals.plos.org/plosbiology/article/file?id=10.1371/journal.pbio.1000547&type=printable P. H. Alvarenga "The Function and Three-Dimensional Structure of a Thromboxane A2/Cysteinyl Leukotriene-Binding Protein from the Saliva of a Mosquito Vector of the Malaria Parasite" PLoS Biol. 2010 Nov 30;8(11):e1000547. doi: 10.1371/journal.pbio.1000547.]</ref> | |<ref name="Alvarenga2010”>[http://journals.plos.org/plosbiology/article/file?id=10.1371/journal.pbio.1000547&type=printable P. H. Alvarenga "The Function and Three-Dimensional Structure of a Thromboxane A2/Cysteinyl Leukotriene-Binding Protein from the Saliva of a Mosquito Vector of the Malaria Parasite" PLoS Biol. 2010 Nov 30;8(11):e1000547. doi: 10.1371/journal.pbio.1000547.]</ref> | ||
|- | |- | ||
| Line 144: | Line 156: | ||
Note: "In solution, it undergoes rapid hydrolysis to form TXB2, a stable but physiologically inactive compound." - therefore they used stable analogues. | Note: "In solution, it undergoes rapid hydrolysis to form TXB2, a stable but physiologically inactive compound." - therefore they used stable analogues. | ||
|[3H]IONO NT-126, a TXA z antagonist, | |[3H]IONO NT-126, a TXA z antagonist, | ||
| + | |36 | ||
|<ref name="Nakahata1992”>[http://ac.els-cdn.com/S0006899310800137/1-s2.0-S0006899310800137-main.pdf?_tid=b2ab095e-155f-11e7-94f0-00000aab0f26&acdnat=1490888822_58d1d45dc3238dede94e4a2f15cada82 N Nakahata et al. "The Presence of Thromboxane A2 Receptors in Cultured Astrocytes From Rabbit Brain" Brain Res 583 (1-2), 100-104. 1992 Jun 26]</ref> | |<ref name="Nakahata1992”>[http://ac.els-cdn.com/S0006899310800137/1-s2.0-S0006899310800137-main.pdf?_tid=b2ab095e-155f-11e7-94f0-00000aab0f26&acdnat=1490888822_58d1d45dc3238dede94e4a2f15cada82 N Nakahata et al. "The Presence of Thromboxane A2 Receptors in Cultured Astrocytes From Rabbit Brain" Brain Res 583 (1-2), 100-104. 1992 Jun 26]</ref> | ||
|- | |- | ||
| Line 152: | Line 165: | ||
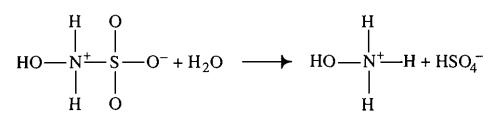
|Hydroxysulfamic acid | |Hydroxysulfamic acid | ||
[[File:HSA.PNG |center|500px]] | [[File:HSA.PNG |center|500px]] | ||
| + | |16 | ||
|<ref name="Littlejohn1989”>[http://www.nrcresearchpress.com/doi/pdf/10.1139/v89-243 D. LITTLEJOHN "The dissociation constant and acid hydrolysis rate of hydroxysulfamic acid" Can. J. Chem. 67, 1596 (1989).]</ref> | |<ref name="Littlejohn1989”>[http://www.nrcresearchpress.com/doi/pdf/10.1139/v89-243 D. LITTLEJOHN "The dissociation constant and acid hydrolysis rate of hydroxysulfamic acid" Can. J. Chem. 67, 1596 (1989).]</ref> | ||
|- | |- | ||
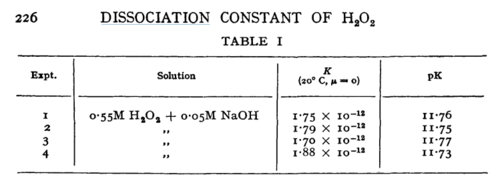
| Line 159: | Line 173: | ||
In vitro | In vitro | ||
|H2O2 | |H2O2 | ||
| + | |16 | ||
|<ref name="Littlejohn1989”>[http://pubs.rsc.org/-/content/articlepdf/1949/tf/tf9494500224 M. G. EVAN "The dissociation constant and acid hydrolysis rate of hydroxysulfamic acid" Trans. Faraday Soc., 1949,45, 224-230]</ref> | |<ref name="Littlejohn1989”>[http://pubs.rsc.org/-/content/articlepdf/1949/tf/tf9494500224 M. G. EVAN "The dissociation constant and acid hydrolysis rate of hydroxysulfamic acid" Trans. Faraday Soc., 1949,45, 224-230]</ref> | ||
|- | |- | ||
| Line 165: | Line 180: | ||
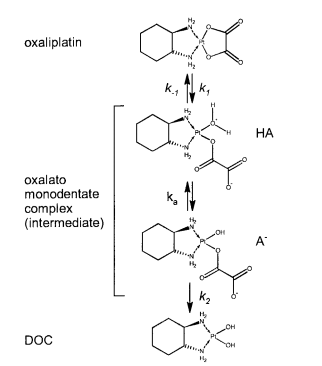
|Oxaliplatin | |Oxaliplatin | ||
|[[File:Oxaliplatin.PNG |center|500px]] | |[[File:Oxaliplatin.PNG |center|500px]] | ||
| + | |16 | ||
|<ref name="Jerremalm”>[http://www.sciencedirect.com/science/article/pii/S0022354916311649 Elin Jerremalm "Hydrolysis of Oxaliplatin—Evaluation of the Acid Dissociation Constant for the Oxalato Monodentate Complex" Journal of Pharmaceutical Sciences Volume 92, Issue 2, February 2003, Pages 436–438]</ref> | |<ref name="Jerremalm”>[http://www.sciencedirect.com/science/article/pii/S0022354916311649 Elin Jerremalm "Hydrolysis of Oxaliplatin—Evaluation of the Acid Dissociation Constant for the Oxalato Monodentate Complex" Journal of Pharmaceutical Sciences Volume 92, Issue 2, February 2003, Pages 436–438]</ref> | ||
|- | |- | ||
Revision as of 10:36, 22 May 2019
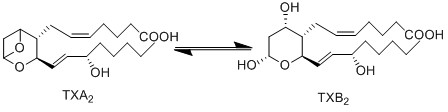
Thromboxane A2 is a bioactive molecule which affects vasoactivity and promotes thrombosis. It is unstable due to the epoxide functional group, and as a consequence has a short half-life of 20- 30 seconds. The hydrolysis reaction results in the generation of biologically inactive TXB2.
Contents
Reaction
Chemical equation
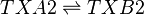
Rate equation
Parameters
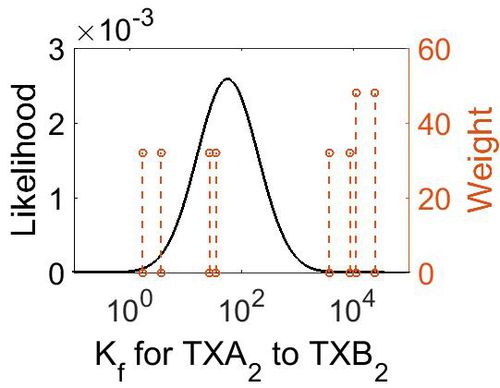
Association Rate Constant (Kf)
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| 3.7e3 ± 0.1e3 | M-1 s-1 | NaCl04 (0.1 - 0.2 M)
Temperature: 25°C |
3 | 32 | [1] |
| 8.7e3 | M-1 s-1 | KCl (1 M)
Temperature: 25°C |
3 | 32 | [1] |
| 1.1e4 ± 0.1e4 | M-1 s-1 | NaCl04 (0.1 - 0.2 M)
Temperature: 25°C |
4 | 32 | [1] |
| 2.4 e4 | M-1 s-1 | KCl (1M)
Temperature: 25°C |
4 | 32 | [1] |
| 3.7e3 ± 0.1e3 | M-1 s-1 | NaCl04 (0.1 - 0.2 M)
Temperature: 25°C |
5 | 32 | [1] |
| 3.6 ± 0.2 | M-1 s-1 | NaCl04 (0.1 - 0.2 M)
Temperature: 25°C |
6 | 32 | [1] |
| 1.7 ± 0.1 | M-1 s-1 | NaCl04 (0.1 - 0.2 M)
Temperature: 25°C |
7 | 32 | [1] |
| 26.7 ± 0.9 | M-1 s-1 | NaCl04 (0.1 - 0.2 M)
Temperature: 25°C |
8 | 32 | [1] |
| 35 | M-1 s-1 | KCl (1M)
Temperature: 25°C |
8 | 32 | [1] |
| 2.25 ± 0.12 | M-1 min-1 | 25°C, in imidazole buffer and also in phosphate buffers, | CO2 to H2CO3 | 32 | [2] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 3.70E+03 | 3.44E+01 | 1.10E+01 | 1.66E+00 |
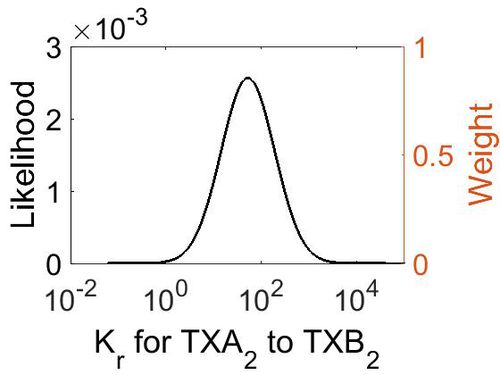
Dissociation Rate Constant (Kr)
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 5.28E+01 | 5.63E+00 | 1.29E+00 |
Dissociation Constant
| Value | Units | Conditions | Substrate | Weight | Reference |
|---|---|---|---|---|---|
| 0.000038 | mM | Temperature: 35°C
Vector:Mosquito Note: "In solution, it undergoes rapid hydrolysis to form TXB2, a stable but physiologically inactive compound." - therefore they used stable analogues. |
carbocyclic TXA2 (analogue of TXA2) | 24 | [3] |
| 0.00000023 | mM | Temperature: 35°C
Vector:Rabbit cultured astrocytes Note: "In solution, it undergoes rapid hydrolysis to form TXB2, a stable but physiologically inactive compound." - therefore they used stable analogues. |
[3H]IONO NT-126, a TXA z antagonist, | 36 | [4] |
| 1500 ± 500 (excluded) | mM | Temperature: 25°C
In vitro |
Hydroxysulfamic acid | 16 | [5] |
| N/A | Temperature: 20°C
In vitro |
H2O2 | 16 | [5] | |
| 5.9 E-8 | N/A | Oxaliplatin | 16 | [6] |
| Mode (M-1 s-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 2.35E-07 | 1.76E+04 | -6.86E+00 | 2.90E+00 |
Related Reactions
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 A. Ross "Vinyl epoxide hydrolysis reactions" J. Am. Chem. Soc., 1982, 104 (6), pp 1658–1665
- ↑ B. Gibbons "Rate of Hydration of Carbon Dioxide and Dehydration of Carbonic Acid at 25" J Biol Chem. 1963 Oct;238:3502-7
- ↑ P. H. Alvarenga "The Function and Three-Dimensional Structure of a Thromboxane A2/Cysteinyl Leukotriene-Binding Protein from the Saliva of a Mosquito Vector of the Malaria Parasite" PLoS Biol. 2010 Nov 30;8(11):e1000547. doi: 10.1371/journal.pbio.1000547.
- ↑ N Nakahata et al. "The Presence of Thromboxane A2 Receptors in Cultured Astrocytes From Rabbit Brain" Brain Res 583 (1-2), 100-104. 1992 Jun 26
- ↑ 5.0 5.1 D. LITTLEJOHN "The dissociation constant and acid hydrolysis rate of hydroxysulfamic acid" Can. J. Chem. 67, 1596 (1989). Cite error: Invalid
<ref>tag; name "Littlejohn1989.E2.80.9D" defined multiple times with different content - ↑ Elin Jerremalm "Hydrolysis of Oxaliplatin—Evaluation of the Acid Dissociation Constant for the Oxalato Monodentate Complex" Journal of Pharmaceutical Sciences Volume 92, Issue 2, February 2003, Pages 436–438