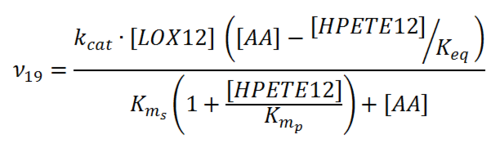Difference between revisions of "Transformation of AA to 12-HPETE"
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | [[Welcome to the In-Silico Model of Cutaneous Lipids Wiki | Return to overview]] | ||
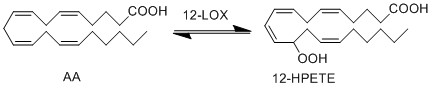
| − | + | The 12- LOX enzyme catalyses the addition of O2 at the C-12 position of AA, producing 12-HPETE. The formation of the unstable hydroperoxy fatty acids (HPETE) begins with the abstraction of a hydrogen radical at the allylic position between two double bonds. The structure undergoes a rearrangement reaction which results in the formation of a conjugated diene system. The insertion of molecular oxygen and a hydrogen leads to the formation of the final structure, a hydroperoxy fatty acid. | |
== Reaction == | == Reaction == | ||
| Line 9: | Line 9: | ||
==Chemical equation== | ==Chemical equation== | ||
| − | <center><math> AA \rightleftharpoons | + | <center><math> AA \rightleftharpoons 12-HPETE </math></center> |
== Rate equation == | == Rate equation == | ||
| Line 15: | Line 15: | ||
[[File:R19.PNG|center|500px]] | [[File:R19.PNG|center|500px]] | ||
| − | == | + | == Enzyme Parameters == |
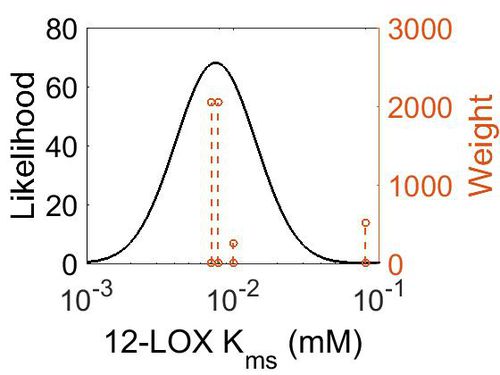
| − | + | === K<sub>ms</sub>=== | |
{|class="wikitable sortable" | {|class="wikitable sortable" | ||
| − | |+ style="text-align: left;" | | + | |+ style="text-align: left;" | Literature values |
|- | |- | ||
! Value | ! Value | ||
| Line 24: | Line 24: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 33: | Line 34: | ||
pH: 7.4 | pH: 7.4 | ||
Temperature: 37°C. | Temperature: 37°C. | ||
| + | |2048 | ||
|<ref name="Lagarde1984"> [http://www.ncbi.nlm.nih.gov/pubmed/6433902 Lagarde M. "Subcellular localization and some properties of lipoxygenase activity in human blood platelets.'' Biochem J. 1984 Sep 1;222(2):495-500.]</ref> | |<ref name="Lagarde1984"> [http://www.ncbi.nlm.nih.gov/pubmed/6433902 Lagarde M. "Subcellular localization and some properties of lipoxygenase activity in human blood platelets.'' Biochem J. 1984 Sep 1;222(2):495-500.]</ref> | ||
|- | |- | ||
| Line 42: | Line 44: | ||
pH: 7 | pH: 7 | ||
Temperature: 24 | Temperature: 24 | ||
| + | |512 | ||
|<ref name="Hada1991"> [http://www.ncbi.nlm.nih.gov/pubmed/1851637 Hada T. "Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins.'' Biochim Biophys Acta. 1991 Apr 24;1083(1):89-93.]</ref> | |<ref name="Hada1991"> [http://www.ncbi.nlm.nih.gov/pubmed/1851637 Hada T. "Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins.'' Biochim Biophys Acta. 1991 Apr 24;1083(1):89-93.]</ref> | ||
|- | |- | ||
| Line 51: | Line 54: | ||
pH: 8 | pH: 8 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |256 | ||
|<ref name="Chen1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8319693 Chen X. S. "Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system.'' Eur J Biochem. 1993 Jun 15;214(3):845-52.]</ref> | |<ref name="Chen1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8319693 Chen X. S. "Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system.'' Eur J Biochem. 1993 Jun 15;214(3):845-52.]</ref> | ||
|- | |- | ||
| Line 60: | Line 64: | ||
pH: 7.4 | pH: 7.4 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |2048 | ||
|<ref name="Romano1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8250832 Romano M. "Lipoxin synthase activity of human platelet 12-lipoxygenase.'' Biochem J. 1993 Nov 15;296 ( Pt 1):127-33.]</ref> | |<ref name="Romano1993"> [http://www.ncbi.nlm.nih.gov/pubmed/8250832 Romano M. "Lipoxin synthase activity of human platelet 12-lipoxygenase.'' Biochem J. 1993 Nov 15;296 ( Pt 1):127-33.]</ref> | ||
|- | |- | ||
|} | |} | ||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the 12-LOX Kms distribution | ||
| + | ! Mode (mM) !! Confidence Interval !! Location parameter (σ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 7.60E-03 || 4.34E+00 || -4.48E+00 || 6.30E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:61.jpg|none|thumb|500px|The estimated probability distribution for 12-LOX Kms. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
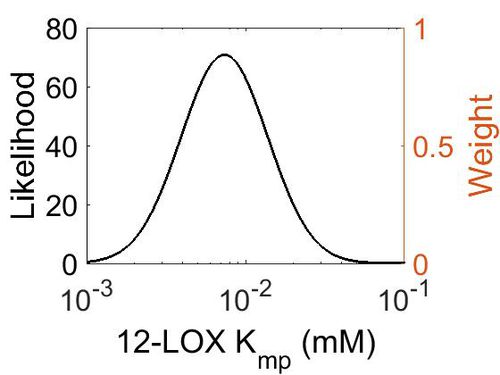
| + | ===K<sub>mp</sub>=== | ||
| + | This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail[[Quantification of parameter uncertainty | here]]). As a result, no confidence interval factor or literature values were cited for this parameter. | ||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the 12-LOX Kmp distribution | ||
| + | ! Mode (mM) !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 7.30E-03 || -4.52E+00 || 6.28E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:62.jpg|none|thumb|500px|The estimated probability distribution for 12-LOX Kmp. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
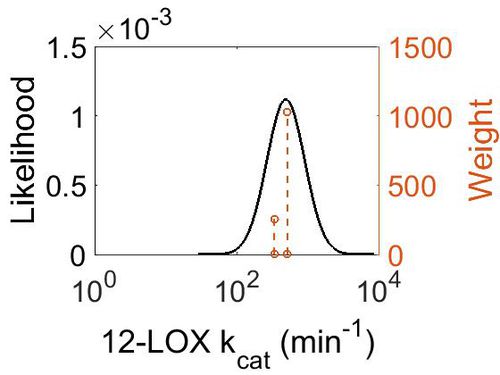
| + | ===k<sub>cat</sub>=== | ||
{|class="wikitable sortable" | {|class="wikitable sortable" | ||
| − | |+ style="text-align: left;" | | + | |+ style="text-align: left;" | Literature values |
|- | |- | ||
! Value | ! Value | ||
| Line 71: | Line 97: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 80: | Line 107: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 25 | Temperature: 25 | ||
| + | |256 | ||
|<ref name="Wecksler2009"> [www.ncbi.nlm.nih.gov/pubmed/19469483 Wecksler A. "Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid'' Biochemistry, 2009, 48 (26), pp 6259–6267]</ref> | |<ref name="Wecksler2009"> [www.ncbi.nlm.nih.gov/pubmed/19469483 Wecksler A. "Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid'' Biochemistry, 2009, 48 (26), pp 6259–6267]</ref> | ||
|- | |- | ||
| Line 89: | Line 117: | ||
pH: 7.4 | pH: 7.4 | ||
Temperature: 37 | Temperature: 37 | ||
| + | |1024 | ||
|<ref name="Richards1997"> [http://pubs.acs.org/doi/abs/10.1021/bi963051a Richards K. "Leukocyte 12-Lipoxygenase: Expression, Purification, and Investigation of the Role of Methionine Residues in Turnover-Dependent Inactivation and 5,8,11,14-Eicosatetraynoic Acid Inhibition'' Biochemistry, 1997, 36 (22), pp 6692–6699]</ref> | |<ref name="Richards1997"> [http://pubs.acs.org/doi/abs/10.1021/bi963051a Richards K. "Leukocyte 12-Lipoxygenase: Expression, Purification, and Investigation of the Role of Methionine Residues in Turnover-Dependent Inactivation and 5,8,11,14-Eicosatetraynoic Acid Inhibition'' Biochemistry, 1997, 36 (22), pp 6692–6699]</ref> | ||
|- | |- | ||
|} | |} | ||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the 12-LOX kcat distribution | ||
| + | ! Mode (min-1) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 4.87E+02 || 1.20E+00 || 6.22E+00 || 1.80E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:63.jpg|none|thumb|500px|The estimated probability distribution for 12-LOX kcat. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
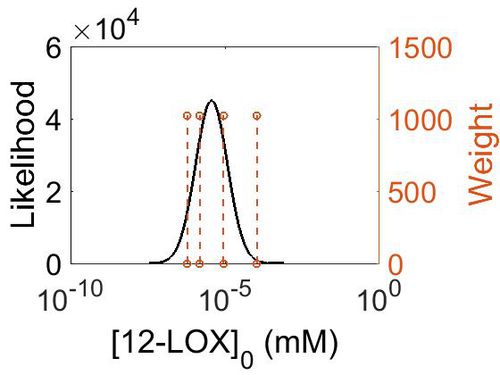
| + | === Enzyme concentration === | ||
| + | |||
| + | To convert the enzyme concentration from ppm to mM, the following [[Common equations#Enzyme concentration (mM)|equation]] was used. | ||
{|class="wikitable sortable" | {|class="wikitable sortable" | ||
| Line 101: | Line 142: | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 110: | Line 152: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | |<ref name="Wilhelm2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13319.pdf M. Wilhelm ''Mass-spectrometry-based draft of the human proteome'' Nature, 2014 509, 582–587]</ref> | ||
|- | |- | ||
| Line 119: | Line 162: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 128: | Line 172: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
| Line 137: | Line 182: | ||
pH: 7.5 | pH: 7.5 | ||
Temperature: 37 °C | Temperature: 37 °C | ||
| + | |1024 | ||
|<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | |<ref name="Kim2014"> [http://www.nature.com/nature/journal/v509/n7502/pdf/nature13302.pdf M. Kim ''A draft map of the human proteome'' Nature, 2014 509, 575–581]</ref> | ||
|- | |- | ||
|} | |} | ||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the 12-LOX concentration distribution | ||
| + | ! Mode (ppm) !! Mode (mM) !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 4.98E-01 ||2.76E-06 || 7.23E+00 || 7.12E-01 || 1.19E+00 | ||
| + | |} | ||
| + | |||
| + | [[Image:156.jpg|none|thumb|500px|The estimated probability distribution for 12-LOX concentration. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
| + | |||
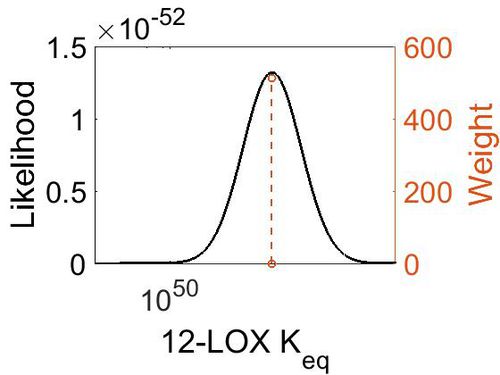
| + | ===K<sub>eq</sub>=== | ||
{|class="wikitable sortable" | {|class="wikitable sortable" | ||
| − | |+ style="text-align: left;" | | + | |+ style="text-align: left;" | Literature values |
|- | |- | ||
| − | ! | + | ! Gibbs Free Energy Change |
! Units | ! Units | ||
! Species | ! Species | ||
! Notes | ! Notes | ||
| + | ! Weight | ||
! Reference | ! Reference | ||
|- | |- | ||
| Line 159: | Line 216: | ||
pH: 7.3 | pH: 7.3 | ||
ionic strength: 0.25 | ionic strength: 0.25 | ||
| + | |64 | ||
|<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=ARACHIDONATE-12-LIPOXYGENASE-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | |<ref name="MetaCyc”>[http://metacyc.org/META/NEW-IMAGE?type=REACTION&object=ARACHIDONATE-12-LIPOXYGENASE-RXN Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471]</ref> | ||
|} | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+ style="text-align: left;" | Description of the 12-LOX Keq distribution | ||
| + | ! Mode !! Confidence Interval !! Location parameter (µ) !! Scale parameter (σ) | ||
| + | |- | ||
| + | | 2.27E+51 || 1.00E+01 || 1.19E+02 || 8.90E-01 | ||
| + | |} | ||
| + | |||
| + | [[Image:64.jpg|none|thumb|500px|The estimated probability distribution for 12-LOX Keq. The value and weight of the literature values used to define the distribution are indicated by an orange dashed line. The x axis is plotted on a log-scale. ]] | ||
== References == | == References == | ||
Latest revision as of 09:18, 21 August 2019
The 12- LOX enzyme catalyses the addition of O2 at the C-12 position of AA, producing 12-HPETE. The formation of the unstable hydroperoxy fatty acids (HPETE) begins with the abstraction of a hydrogen radical at the allylic position between two double bonds. The structure undergoes a rearrangement reaction which results in the formation of a conjugated diene system. The insertion of molecular oxygen and a hydrogen leads to the formation of the final structure, a hydroperoxy fatty acid.
Contents
Reaction
Chemical equation
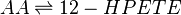
Rate equation
Enzyme Parameters
Kms
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 7.20E-03 | 
|
Human | Expression Vector: Platelet
Enzyme: 12-Lipoxygenase pH: 7.4 Temperature: 37°C. |
2048 | [1] |
| 8.00E-02 | 
|
Human | Expression Vector: Platelet
Enzyme: 12-Lipoxygenase pH: 7 Temperature: 24 |
512 | [2] |
| 1.00E-02 | 
|
Human Platlet | Expression Vector: Baculovirus
Enzyme: 12-Lipoxygenase pH: 8 Temperature: 37 |
256 | [3] |
| 7.90E-03 ± 8.00E-04 | 
|
Human | Expression Vector: Platelets
Enzyme:12-Lipoxygenase pH: 7.4 Temperature: 37 |
2048 | [4] |
| Mode (mM) | Confidence Interval | Location parameter (σ) | Scale parameter (σ) |
|---|---|---|---|
| 7.60E-03 | 4.34E+00 | -4.48E+00 | 6.30E-01 |
Kmp
This is a “Dependent parameter”, meaning that the log-normal distribution for this parameter was calculated using multivariate distributions (this is discussed in detail here). As a result, no confidence interval factor or literature values were cited for this parameter.
| Mode (mM) | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|
| 7.30E-03 | -4.52E+00 | 6.28E-01 |
kcat
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 336 ± 12 | per minute | Human | Expression Vector: Human reticulocyte
Enzyme: 15-lipoxygenase-1 pH: 7.5 Temperature: 25 |
256 | [5] |
| 504 | per minute | Wild Boar | Expression Vector: E. coli.
Enzyme: 12-Lipoxygenase pH: 7.4 Temperature: 37 |
1024 | [6] |
| Mode (min-1) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 4.87E+02 | 1.20E+00 | 6.22E+00 | 1.80E-01 |
Enzyme concentration
To convert the enzyme concentration from ppm to mM, the following equation was used.
| Value | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| 19.8 | 
|
Human | Expression Vector: Spleen
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [7] |
| 1.60 | 
|
Human | Expression Vector: Liver
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| 0.28 | 
|
Human | Expression Vector: Gut
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| 0.11 | 
|
Human | Expression Vector: Pancreas
Enzyme: 12-LOX pH: 7.5 Temperature: 37 °C |
1024 | [8] |
| Mode (ppm) | Mode (mM) | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|---|
| 4.98E-01 | 2.76E-06 | 7.23E+00 | 7.12E-01 | 1.19E+00 |
Keq
| Gibbs Free Energy Change | Units | Species | Notes | Weight | Reference |
|---|---|---|---|---|---|
| (-69.979996) | kcal/mol | Not stated | Estimated
Enzyme: 12-LOX Substrate: Arachidonate Product: 12-HPETE pH: 7.3 ionic strength: 0.25 |
64 | [9] |
| Mode | Confidence Interval | Location parameter (µ) | Scale parameter (σ) |
|---|---|---|---|
| 2.27E+51 | 1.00E+01 | 1.19E+02 | 8.90E-01 |
References
- ↑ Lagarde M. "Subcellular localization and some properties of lipoxygenase activity in human blood platelets. Biochem J. 1984 Sep 1;222(2):495-500.
- ↑ Hada T. "Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins. Biochim Biophys Acta. 1991 Apr 24;1083(1):89-93.
- ↑ Chen X. S. "Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system. Eur J Biochem. 1993 Jun 15;214(3):845-52.
- ↑ Romano M. "Lipoxin synthase activity of human platelet 12-lipoxygenase. Biochem J. 1993 Nov 15;296 ( Pt 1):127-33.
- ↑ [www.ncbi.nlm.nih.gov/pubmed/19469483 Wecksler A. "Mechanistic Investigations of Human Reticulocyte 15- and Platelet 12-Lipoxygenases with Arachidonic Acid Biochemistry, 2009, 48 (26), pp 6259–6267]
- ↑ Richards K. "Leukocyte 12-Lipoxygenase: Expression, Purification, and Investigation of the Role of Methionine Residues in Turnover-Dependent Inactivation and 5,8,11,14-Eicosatetraynoic Acid Inhibition Biochemistry, 1997, 36 (22), pp 6692–6699
- ↑ M. Wilhelm Mass-spectrometry-based draft of the human proteome Nature, 2014 509, 582–587
- ↑ 8.0 8.1 8.2 M. Kim A draft map of the human proteome Nature, 2014 509, 575–581
- ↑ Caspi et al 2014, "The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases," Nucleic Acids Research 42:D459-D471