Difference between revisions of "Serine out"
(→Parameters with uncertainty) |
|||
| Line 34: | Line 34: | ||
==Parameters with uncertainty== | ==Parameters with uncertainty== | ||
*The transport rates have been modelled using mass action kinetics (i.e., as non-saturable, non-enzymatic reactions). No information is available about the uncertainty of these parameters. As these parameters are strictly positive, they are sampled using a log-normal distribution as are and values. The means of <math>K_{1}</math> are set to the value reported in Turnaev (2006) et al. <ref name="Turnaev_2006"></ref> for the fixed-parameter model. The reaction is presumed to be at equilibrium, such that <math>K_{eq} = 1</math> and <math>K_{1} = K_{2}</math>. The sampling of the parameters are done in a way so that it ranges between <math>[0.001\times mean \quad 1000 \times mean ]</math> to allow a large exploration of the parameter space. | *The transport rates have been modelled using mass action kinetics (i.e., as non-saturable, non-enzymatic reactions). No information is available about the uncertainty of these parameters. As these parameters are strictly positive, they are sampled using a log-normal distribution as are and values. The means of <math>K_{1}</math> are set to the value reported in Turnaev (2006) et al. <ref name="Turnaev_2006"></ref> for the fixed-parameter model. The reaction is presumed to be at equilibrium, such that <math>K_{eq} = 1</math> and <math>K_{1} = K_{2}</math>. The sampling of the parameters are done in a way so that it ranges between <math>[0.001\times mean \quad 1000 \times mean ]</math> to allow a large exploration of the parameter space. | ||
| − | |||
| − | |||
{|class="wikitable" | {|class="wikitable" | ||
! Parameter | ! Parameter | ||
| Line 50: | Line 48: | ||
|Sampled between 0.0000103 and 10.3 | |Sampled between 0.0000103 and 10.3 | ||
|} | |} | ||
| − | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 11:34, 15 May 2014
This reaction describes the utilization of the endproduct Serine in other pathways.
Contents
Reaction equation
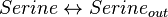
Rate equation
Simple reversible mass action rate law is used.
![v = K_{1} * [Serine] - K_{2} * [Serine_{out}]](/wiki/images/math/e/6/b/e6b7d292763cb679cbd1de648b4b0ddf.png)
Parameters
- For Transport reactions it is presumed to be at equilibrium, such that
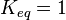 and
and 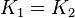 .
.
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|
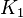
|
0.0103 [1] | 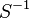
|
Escherichia coli | |
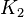
|
0.0103 | 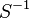
|
Parameters with uncertainty
- The transport rates have been modelled using mass action kinetics (i.e., as non-saturable, non-enzymatic reactions). No information is available about the uncertainty of these parameters. As these parameters are strictly positive, they are sampled using a log-normal distribution as are and values. The means of
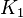 are set to the value reported in Turnaev (2006) et al. [1] for the fixed-parameter model. The reaction is presumed to be at equilibrium, such that
are set to the value reported in Turnaev (2006) et al. [1] for the fixed-parameter model. The reaction is presumed to be at equilibrium, such that 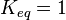 and
and 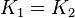 . The sampling of the parameters are done in a way so that it ranges between
. The sampling of the parameters are done in a way so that it ranges between ![[0.001\times mean \quad 1000 \times mean ]](/wiki/images/math/7/0/5/70565b13097c64cb12ee455eb2006ee7.png) to allow a large exploration of the parameter space.
to allow a large exploration of the parameter space.
| Parameter | Value | Organism | Remarks |
|---|---|---|---|
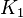
|
Sampled between 0.0000103 and 10.3 | HeLa cell line | |
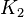
|
Sampled between 0.0000103 and 10.3 |