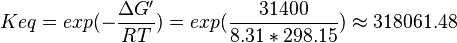Difference between revisions of "Pyruvate kinase"
(→Parameters with uncertainty) |
(→Parameters with uncertainty) |
||
| Line 82: | Line 82: | ||
<imagemap> | <imagemap> | ||
| − | Image:Allosteric_L_val.png|frameless|center|alt=Allosteric L Value | + | Image:Allosteric_L_val.png|frameless|center|600px|alt=Allosteric L Value |
</imagemap> | </imagemap> | ||
Latest revision as of 15:30, 26 May 2015
Pyruvate kinase is a transferase enzyme that catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to ADP, yielding one molecule of pyruvate and one molecule of ATP.
Contents
Chemical reaction
Rate equation
The rate equation is represented by the allosteric regualation model of Monod, Wyman and Changeux (MWS). Fru1,6BP and Serine are activators and ATP is inhibiting. Simple Micahelis-Menten kinetics (Briggs Haldane) is used for ADP and reverse reaction [1]
![v=V_m \left( \left(\frac{\frac{[ADP]}{K_{ADP}}}{1+\frac{[ADP]}{K_{ADP}}}\right) \left( \frac{\frac{[PEP]}{Km_{PEP}}\left( 1+\frac{[PEP]}{Km_{PEP}} \right)^3 }{ \frac{L \left( 1 + \frac{[ATP]}{Ki_{ATP}} \right)^4 }{ \left( 1 + \frac{[SER]}{Ka_{SER}} \right)^4 \left( 1 + \frac{F1,6BP}{Ka_{F1,6BP}} \right)^4 } + \left( 1 + \frac{[PEP]}{Km_{PEP}} \right)^4} \right) - \left( \frac{\frac{[ATP][PYR]}{K_{ATP} \times K_{PYR} \times K_{eq}}}{1 +\frac{[ATP]}{K_{ATP}} + \frac{[PYR]}{K_{PYR}} + \frac{[ATP][PYR]}{K_{ATP} \times K_{PYR} }} \right) \right)](/wiki/images/math/6/0/8/608585715d214881c663ba97b3810b40.png)
Parameter values
- The dissociation constant is commonly used to describe the affinity between a ligand (L) (such as a drug) and a protein (P) i.e. how tightly a ligand binds to a particular protein. In the specific case of antibodies (Ab) binding to antigen (Ag), usually the affinity constant is used. It is the inverted dissociation constant.
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
1.9[2] | 
|
HeLa cell line | |
 [2] [2]
|
195172 | Recalculated from the ΔGº´ = - 31.4 KJ mol-1. | ||
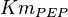 [2] [2]
|
0.014 | mM | ||
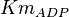 [2] [2]
|
0.4 | mM | ||
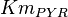 [3] [3]
|
10 | mM | ||
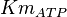 [3] [3]
|
0.86 | mM | ||
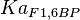 [4] [4]
|
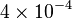
|
mM | ||
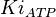 [4] [4]
|
2.5 | mM | ||
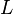 [5] [5]
|
1 | Dimensionless | ||
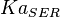
|
5 | mM | For allosteric regulation the affinity constant is used. It is the inverted dissociation constant. so 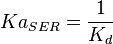 where where 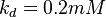 [6] [6]
|
Parameters with uncertainty
- Three values of
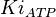 have been reported as 0.14 mM, 0.12 mM, 0.33 mM in Boyer et. al. (1969) [7]. However, he also cited papers that describe the Ki value between 3.5 mM and 1.0 mM. Hernandez et. al. reported a value of 2.5 mM. Considering all these factors we consider the vlaue to lie in between 3.5mM and 1.0 mM. Taking these three values of 1.0, 2.5, 3.5 we have the Mean and Std. Dev. as
have been reported as 0.14 mM, 0.12 mM, 0.33 mM in Boyer et. al. (1969) [7]. However, he also cited papers that describe the Ki value between 3.5 mM and 1.0 mM. Hernandez et. al. reported a value of 2.5 mM. Considering all these factors we consider the vlaue to lie in between 3.5mM and 1.0 mM. Taking these three values of 1.0, 2.5, 3.5 we have the Mean and Std. Dev. as 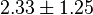 .
.
- Four isoforms of PYK exists. Among those four, three isoforms (R, L and M2) exhibits cooperative kinetics activated by Fru1,6BP (Ka = 0.00006 - 0.0004). For calculating the mean and standard deviation we consider max = 0.0004 and min = 0.00006. The range rule tells that the mean of a sample is the average of the maximum and the minimum value and standard deviation is approximately equal to one fourth of the range of the data ie. s = (Maximum – Minimum)/4. So the mean is (0.0004 + 0.00006)/2= 0.00023 and std. dev. = 0.000085[8].
- Four values for
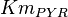 found in the literature are 0.025,0.055 [9] for Sparus aurata liver, 0.48 for Selenomonas ruminantium [10] and 10 [3]. The mean and std. dev. from these 4 values are 2.64 and 4.9.
found in the literature are 0.025,0.055 [9] for Sparus aurata liver, 0.48 for Selenomonas ruminantium [10] and 10 [3]. The mean and std. dev. from these 4 values are 2.64 and 4.9.
- Two values of
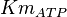 have been reported in the literature. 0.35 for Sparus aurata liver [9] and 0.86 [3]. The uncertainty is then
have been reported in the literature. 0.35 for Sparus aurata liver [9] and 0.86 [3]. The uncertainty is then 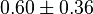 .
.
- Two values of Serine activation for Pyruvate Kinase constant have been reported; 2[11] and 5 [6]. Taking mean and Std. Dev. for this two value gives
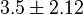 .
.
- Del valle (1986) [12] determined the Allosteric Constant
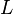 with the effect of L-alanine and Furctose1,6BP. For L-alanine the value of
with the effect of L-alanine and Furctose1,6BP. For L-alanine the value of 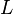 is reported to be
is reported to be 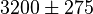 whereas in the presence of
whereas in the presence of 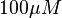 Fru1,6BP the value is reported as
Fru1,6BP the value is reported as 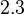 . As in our model we do not consider the affect of L-alaine but consder only the effect of Fru1,6BP we took this value of
. As in our model we do not consider the affect of L-alaine but consder only the effect of Fru1,6BP we took this value of 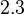 . But there is no uncertainty reported with this value. So we took the same proportion of uncertainty as that of L-alaine for this value. This gives the value as
. But there is no uncertainty reported with this value. So we took the same proportion of uncertainty as that of L-alaine for this value. This gives the value as 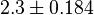
- The
 values found in the literature are ΔGº´ = -23.0 | -31.4 | -24.98 | -26.815 kJ/mol, =>Keq = 10753.64 | 318061.48 | 16190 | 32995
values found in the literature are ΔGº´ = -23.0 | -31.4 | -24.98 | -26.815 kJ/mol, =>Keq = 10753.64 | 318061.48 | 16190 | 32995
- Hernandez reported the value of
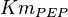 as 0.014 in [2]. But there is not Std. Dev. mentioned with the data. So we calculate the Std. Dev. based on the same maximum ratio of other kinetic parameters Std. Dev. which is for parameter
as 0.014 in [2]. But there is not Std. Dev. mentioned with the data. So we calculate the Std. Dev. based on the same maximum ratio of other kinetic parameters Std. Dev. which is for parameter 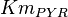 . Considering this ratio of error the value for
. Considering this ratio of error the value for 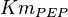 is
is 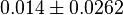 .
.
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
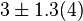 [13] [13] 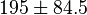 [2] [2]
|
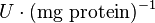 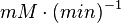
|
HeLa cell line | |
 [2] [2]
|
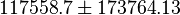
|
|||
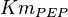
|
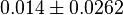 [14] [14]
|
mM | ||
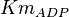
|
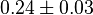 [14] [14]
|
mM | ||
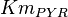
|
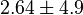
|
mM | ||
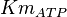
|
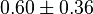
|
mM | ||
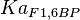
|
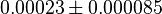
|
mM | ||
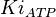
|
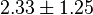 [7] [7]
|
mM | ||
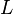
|
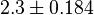 [12] [12]
|
Dimensionless | ||
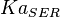
|
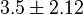
|
mM |
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 318061.48 | pH=7, T=25°C | Lehninger, (2008)[15] p 553:
|
| 10753.64 | pH=7, T=25°C | 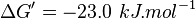 , , 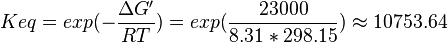
|
| 23860.98 | pH=7, T=25°C | 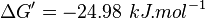 , , 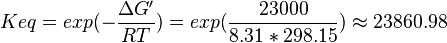
|
- Averaging these values gives
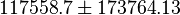
References
- ↑ Monod J, Wyman J, Changeux J-P (1965). On the Nature of Allosteric Transitions: A Plausible Model . Journal of Molecular Biology 12:88–118 (doi)
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ 3.0 3.1 3.2 3.3 H.U. Bergmeyer. Methods of Enzymatic Analysis. Verlag Chemie, Winheim
- ↑ 4.0 4.1 Imamura K, Tanaka T (1982). Pyruvate kinase isoenzymes from rat, Methods Enzymol. 90 (1982) 150–165
- ↑ Arbitrary value
- ↑ 6.0 6.1 Chaneton, B. et al.(2012) Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458–462
- ↑ 7.0 7.1 P.D. Boyer (1969, The inhibition of pyruvate kinase by ATP: A Mg++ buffer system for use in enzyme studies, Biochemical and Biophysical Research Communications, Volume 34, Issue 5, 10 March 1969, Pages 702–706
- ↑ A. Marín-Hernández, J.C. Gallardo-Pérez, S.J. Ralph, S. Rodríguez-Enríquez, R. Moreno-Sánchez (2009), HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms, Mini Rev. Med. Chem., 9, pp. 1084–1101
- ↑ 9.0 9.1 Gomez-Milan E., Cardenete G., Sanchez-Muros M.J. (2007), Annual variations in the specific activity of fructose 1,6-bisphosphatase, alanine aminotransferase and pyruvate kinase in the Sparus aurata liver, Comp. Biochem. Physiol. B 147, 49-55
- ↑ Asanuma N., Hino T. (2001), Molecular characterization, enzyme properties and transcriptional regulation of phosphoenolpyruvate carboxykinase and pyruvate kinase in a ruminal bacterium, Selenomonas ruminantium, Microbiology 147, 681-690 (2001)
- ↑ M.J. Merrins, A.R. Van Dyke, A.K. Mapp, M.A. Rizzo, L.S. Satin (2013), Direct measurements of oscillatory glycolysis in pancreatic islet beta-cells using novel fluorescence resonance energy transfer (FRET) biosensors for pyruvate kinase M2 activity. J. Biol. Chem. 288, 33312–33322
- ↑ 12.0 12.1 del Valle,P.,de Arriaga, D., Busto, F. and Soler, J. (1986) A study of the allosteric kinetics of Phycomyces pyruvate kinase as judged by the effect of e-alanine and fructose 1,6-bisphosphate. Biochim. Biophys. Acta 874, 193-204
- ↑ Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)
- ↑ 14.0 14.1 Dombrauckas, J. D., Santarsiero, B. D. & Mesecar, A. D. (2005) Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44, 9417–9429
- ↑ David L. Nelson, Michael M. Cox (2008), Lehninger Principles of Biochemistry (5th edn), W. H. Freeman and Company
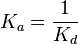


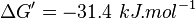 ,
,