Back to terpene model MAIN page.
Back to LimSynth model page.
Kinetic Parameters
Turnover numbers (Kcat)
| Parameter
|
Value
|
Units
|
Description
|
References
|
| kcatforward
|
3.9x10-5
|
s-1
|
Limonene synthase expressed at 37°C, 40µM GPP, Citrus sinensis, product formation 1.17x105 µM/sec

|
[1]
|
| kcatforward
|
0.13
|
s-1
|
Limonene synthase expressed at 20°C, 200µM GPP, Citrus sinensis, pH 7.5
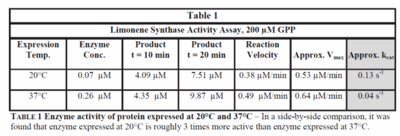
|
[2]
|
| kcatforward
|
0.04
|
s-1
|
Limonene synthase expressed at 37°C, 200µM GPP, Citrus sinensis, pH 7.5

|
[2]
|
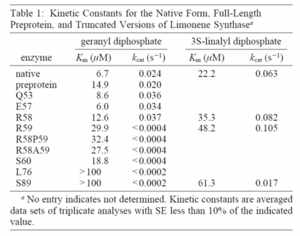
| Kcat
|
0.3
|
s-1
|

|
[3]
|
| Kcat
|
0.020
|
s-1
|

|
[4]
|
| Kcat
|
0.024
|
s-1
|
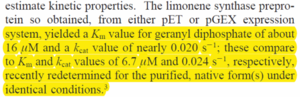
Below is an excerpt from Williams 1998, but data source is from Ogura 1997, describing data from wildtype E.coli

|
[5]
|
| Kcat
|
0.082
|
s-1
|
Kcat value for CsTPS1, Cannabis sativa, pH6.5, 40°C, enzyme assay
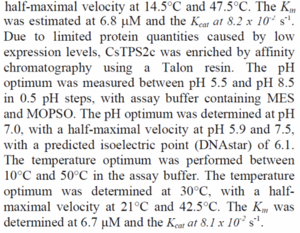
|
[6]
|
Michaelis-Menten constant (Km)
Substrate and Enzyme Concentrations or Amount
| Parameter
|
Value
|
Units
|
Description
|
References
|
| [GPP]
|
40
|
µM
|
Limonene synthase expressed at 37°C, 40µM GPP, Citrus sinensis, product formation 1.17x105 µM/sec

|
[1]
|
| [GPP]
|
10
|
µM
|
Limonene synthase expressed at 37°C, 40µM GPP, Citrus sinensis, product formation 1.17x105 µM/sec, [E]: 0.3 µM

|
[1]
|
| [LimSynth]
|
0.3
|
µM
|
Limonene synthase expressed at 37°C, 10µM GPP, Citrus sinensis, product formation 1.17x105 µM/sec, [E]: 0.3 µM

|
[1]
|
| [LimSynth]
|
0.07
|
µM
|
Limonene synthase expressed at 20°C, 200µM GPP, Citrus sinensis

|
[2]
|
| [LimSynth]
|
0.26
|
µM
|
Limonene synthase expressed at 37°C, 200µM GPP, Citrus sinensis, pH 7.5

|
[2]
|
| Enzyme amount
|
1-10
|
µg
|

|
[3]
|
| Enzyme amount
|
10-20
|
µg
|

|
[8]
|
| [GPP]
|
10
|
µM
|

|
[3]
|
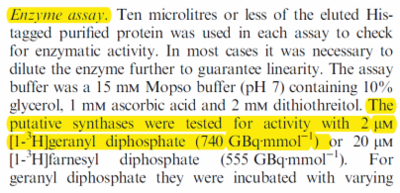
| [GPP]
|
2
|
µM
|
|
[7]
|
| [GPP]
|
0.1
|
µM
|
0.1 -100 µM

|
[7]
|
| [GPP]
|
100
|
µM
|
0.1 -100 µM

|
[7]
|
| [LimSynth]
|
1 - 50
|
µg
|
Amount of native or recombinant protein (E.coli) used for Limonene synthase enzyme assay

|
[4]
|
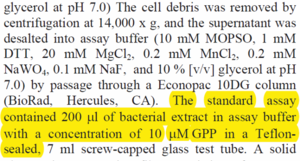
| [GPP]
|
1 - 40
|
µM
|
Substrate concentration range for enzyme assay

|
[4]
|
| [GPP]
|
10
|
µM
|
|
[6]
|
| Enzyme Amount (Possibly)
|
1.25
|
µg
|
Possible it is referring to enzyme amount used for CsTPS1 (limonene producing terpene synthase).

|
[6]
|
| [GPP]
|
25
|
µM
|
GPP concentration used, for preprotein (?) expressed in E.coli.

|
[4]
|
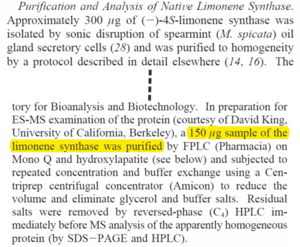
| Enzyme amount
|
150
|
µg
|
Purified (-)-4S-limonene synthase amount.
|
[4]
|
Maximum velocity (Vmax), Enzyme activity & Specific enzyme activity
| Parameter
|
Value
|
Units
|
Description
|
References
|
| Vmaxforward
|
0.53
|
µM/min
|
Limonene synthase expressed at 20°C, 200µM GPP, Citrus sinensis, pH7.5

|
[2]
|
| Vmaxforward
|
0.64
|
µM/min
|
Limonene synthase expressed at 37°C, 200µM GPP

|
[2]
|
| Specific enzyme activity
|
19
|
µmol/h/mg
|

|
[3]
|
Other information
| Parameter
|
Value
|
Units
|
Description
|
References
|
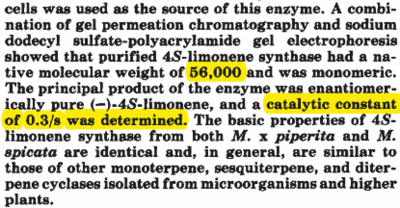
| Limonene Synthase
Molecular Weight
|
56 kDa
|
kDa
|
Limonene synthase purification
|
[3]
|
Back to the main model page.
References
- ↑ 1.0 1.1 1.2 1.3 Olsen, S. N. 2011. "Isolation, Purification, and Characterization of (+)-4R-limonene synthase: The first step in exploring enzyme stereospecificity in terpenoid biosynthesis", Masters Thesis, Brandeis Univeristy
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Entova, S. 2013. "Kinetic characterization, crystallization, and photosynthetic expression of (+)-4R-limonene synthase from C. sinensis", Masters Thesis, Brandeis Univeristy
- ↑ 3.0 3.1 3.2 3.3 3.4 Alonso et. al. 1992. "Purification of 4S-Limonene Synthase, a Monoterpene Cyclase from the Glandular Trichomes of Peppermint (Mentha x piperita) and Spearmint (Mentha spicata)", The journal of Biological Chemistry, 267(11):7582-7587
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Williams, D.C. et. al. 1998. [www.ncbi.nlm.nih.gov/pubmed/9724535 "Truncation of Limonene Synthase preprotein provides a fully active 'pseudomature' form of this monoterpene cyclase and reveals the function of the amino-terminal arginine pair ",Biochemistry, 37:12213-12220] Cite error: Invalid
<ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content Cite error: Invalid <ref> tag; name "Williams1998" defined multiple times with different content
- ↑ 5.0 5.1 Ogura, K. & Koyama, T. 1997. "Dynamic Aspects of Natural Products Chemistry", pp 1-23 Cite error: Invalid
<ref> tag; name "Ogura1997" defined multiple times with different content
- ↑ 6.0 6.1 6.2 6.3 Cite error: Invalid
<ref> tag; name "Gunnewich2006" defined multiple times with different content Cite error: Invalid <ref> tag; name "Gunnewich2006" defined multiple times with different content
- ↑ 7.0 7.1 7.2 7.3 Cite error: Invalid
<ref> tag; name "Lucker2002" defined multiple times with different content Cite error: Invalid <ref> tag; name "Lucker2002" defined multiple times with different content Cite error: Invalid <ref> tag; name "Lucker2002" defined multiple times with different content
- ↑ 8.0 8.1 Adam, K. et. al. 1996. "Partial purification and characterization of a monoterpene cyclase, limonene synthase, from the liverwort Ricciocarpos natans. 332(2):pp 352-356.