Difference between revisions of "Lactate dehydrogenase"
(→Parameters with uncertainty) |
(→Equilibrium constant) |
||
| Line 113: | Line 113: | ||
| | | | ||
| <ref name="Brooks_1999"> Brooks GA (1999). ''Are arterial, muscle and working limb lactate exchange data obtained on men at altitude consistent with the hypothesis of an intracellular lactate shuttle?''. Adv Exp Med Biol, 474:185–204 </ref> | | <ref name="Brooks_1999"> Brooks GA (1999). ''Are arterial, muscle and working limb lactate exchange data obtained on men at altitude consistent with the hypothesis of an intracellular lactate shuttle?''. Adv Exp Med Biol, 474:185–204 </ref> | ||
| + | |- | ||
| + | | 0.067 | ||
| + | | pH=7, T=25°C | ||
| + | | Lehninger, (1975)<ref name="lehninger75">Lehninger, A.L. (1975) Biochemistry (2nd edn), Worth</ref> p 407:<br/> | ||
|} | |} | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 11:19, 1 July 2014
A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. Lactate dehydrogenase catalyzes the conversion of pyruvate to lactate and back, as it converts NADH to NAD+ and back.
Contents
Chemical reactions
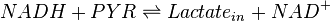
Rate equation
RAndom Bi-Bi reversible Michaelis-Menten equation is used. [1]
![\frac{V_{mf}\frac{[NADH][PYR]}{Km_{NADH} K_{PYR}} - V_{mr}\frac{[Lactate_{in}][NAD]}{Km_{Lactate_{in}} K_{NAD}}}{1 + \frac{[NADH]}{Km_{NADH}} + \frac{[PYR]}{Km_{PYR}} + \frac{[NADH][PYR]}{Km_{NADH} Km_{PYR}} + \frac{[Lactate_{in}][NAD]}{Km_{Lactate_{in}} Km_{NAD}} + \frac{[Lactate_{in}]}{Km_{Lactate_{in}}} + \frac{[PYR]}{Km_{PYR}} }](/wiki/images/math/4/b/2/4b2b29cf3850c4c61d11218132ecd486.png)
Modified rate law to take Thermodynamic constraint into consideration
![\frac{V_{mf}\frac{[NADH][PYR]}{Km_{NADH} K_{PYR}} \left(1 - \frac{[Lactate_{in}][NAD]}{K_{eq}[NADH][PYR]} \right)}{1 + \frac{[NADH]}{Km_{NADH}} + \frac{[PYR]}{Km_{PYR}} + \frac{[NADH][PYR]}{Km_{NADH} Km_{PYR}} + \frac{[Lactate_{in}][NAD]}{Km_{Lactate_{in}} Km_{NAD}} + \frac{[Lactate_{in}]}{Km_{Lactate_{in}}} + \frac{[PYR]}{Km_{PYR}} }](/wiki/images/math/e/5/4/e54419f6b7939faa1e16564482b31638.png)
Prameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
3.4 [1] | 
|
HeLa cell line | |

|
0.54 | 
|
HeLa cell line | |
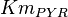
|
0.1 | mM | HeLa cell line | |
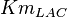
|
4.7 | mM | Rat AS-30D hepatoma | |
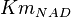
|
0.07 | mM | HeLa cell line | |
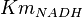
|
0.002 | mM | HeLa cell line |
Parameters with uncertainty
- Mean and Std. Dev. for
 has been reported in Table S3 for Marín-Hernández (2011) et. al. [1]. The Std. Dev. for
has been reported in Table S3 for Marín-Hernández (2011) et. al. [1]. The Std. Dev. for  is calculated based on the same ratio for
is calculated based on the same ratio for  .
.
- The value for
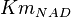 and
and 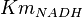 has been reported for Ovine (Sheep) as
has been reported for Ovine (Sheep) as 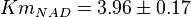 and
and 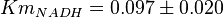 [4]. Due to lack of data in Human cells these two values are considered in our model.
[4]. Due to lack of data in Human cells these two values are considered in our model.
-
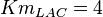 for LDH-1 and 2 isoforms and
for LDH-1 and 2 isoforms and 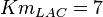 for LDH-4 and 5 isoforms are being reported in Marín-Hernández et. al. (2009)[5]. Mean and Std. Dev. from these two values are
for LDH-4 and 5 isoforms are being reported in Marín-Hernández et. al. (2009)[5]. Mean and Std. Dev. from these two values are 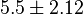
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
Failed to parse (Cannot store math image on filesystem.): 3.4 \pm 0.5 (3) [6] | 
|
HeLa cell line | |

|
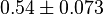
|

|
HeLa cell line | |
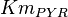
|
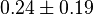
|
mM | HeLa cell line | |
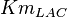
|
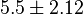
|
mM | Rat AS-30D hepatoma | |
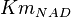
|
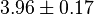
|
mM | Ovine (Sheep) | |
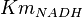
|
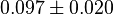
|
mM | Ovine (Sheep) |
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 36000 | [7] | |
| 0.067 | pH=7, T=25°C | Lehninger, (1975)[8] p 407: |
References
- ↑ 1.0 1.1 1.2 1.3 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ LeVan K.M., Goldberg E. (1991), Properties of human testis-specific lactate dehydrogenase expressed from Escherichia coli, Biochem. J. 273, 587-592 (1991)
- ↑ Pettit S.M., Nealon D.A., Henderson A.R. (1981), Purification of lactate dehydrogenase isoenzyme-5 from human liver, Clin. Chem. 27, 88-93 (1981)
- ↑ M. Doughty (1998), Some kinetic properties of lactate dehydrogenase activity in cell extracts from a mammalian (ovine) corneal epithelium, Exp. Eye Res., 66, pp. 231–239
- ↑ A. Marín-Hernández, J.C. Gallardo-Pérez, S.J. Ralph, S. Rodríguez-Enríquez, R. Moreno-Sánchez (2009), HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms, Mini Rev. Med. Chem., 9, pp. 1084–1101
- ↑ Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)
- ↑ Brooks GA (1999). Are arterial, muscle and working limb lactate exchange data obtained on men at altitude consistent with the hypothesis of an intracellular lactate shuttle?. Adv Exp Med Biol, 474:185–204
- ↑ Lehninger, A.L. (1975) Biochemistry (2nd edn), Worth