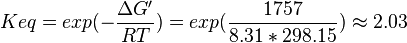Difference between revisions of "Enolase"
(→Rate equation) |
(→Parameters with uncertainty) |
||
| Line 73: | Line 73: | ||
|mM | |mM | ||
|Human muscle | |Human muscle | ||
| + | |} | ||
| + | |||
| + | === Equilibrium constant === | ||
| + | {|class="wikitable" | ||
| + | ! Equilibrium constant | ||
| + | ! Conditions | ||
| + | ! Source | ||
| + | |- | ||
| + | | 3.6 | ||
| + | | pH=7, T=25°C | ||
| + | | Voet et al.<ref name="voet">Voet, D., Voet., J.G. and Pratt, C. W. (1999) Fundamentals of biochemistry, Wiley</ref> from Newshole et al. (1973) <ref name="newshole73">Newshole, E.A. and Stuart, C. (1973) Regulation in Metabolism, Wiley</ref>p 97:<br/> | ||
| + | <math>\Delta G' = -3.2\ kJ.mol^{-1}</math>, <math>Keq = exp(-\frac{\Delta G'}{RT}) = exp(\frac{3200}{8.31*298.15}) \approx 3.6</math> | ||
| + | |- | ||
| + | | 6.7 | ||
| + | | T=25°C | ||
| + | | Bergmeyer ''Methods of enzymatic analysis'' page 449<ref name="bermeyer74">Bergmeyer H.U. (1974) ''Methods of enzymatic analysis'', Publisher: Verlag Chemie (vol 1)</ref> | ||
| + | |- | ||
| + | | 2.03 | ||
| + | | pH=7, T=297.15 K | ||
| + | | From Meyerhof et al. (1947)<ref name="meyerhof49">Meyerhof O. and Oesper P. (1947) J. Biol. Chem. 170(1):1-22 [[http://www.jbc.org/content/170/1.toc J. Biol. Chem.]]</ref>: | ||
| + | <math>\Delta G' = -1.757\ kJ.mol^{-1}</math>, <math>Keq = exp(-\frac{\Delta G'}{RT}) = exp(\frac{1757}{8.31*298.15}) \approx 2.03</math> | ||
| + | |- | ||
| + | | 4.29 | ||
| + | | pH=7, T=298.15 K, c(MgSO4,mol dm-3) =0.001 | ||
| + | | From Wold et al. (1957) (NIST database<ref name="nist">Goldberg R.N., Tewari Y.B. and Bhat T.N. (2004) Bioinformatics 20(16):2874-2877 [[http://www.ncbi.nlm.nih.gov/pubmed?term=15145806 pmid: 15145806]]</ref> [[http://xpdb.nist.gov/enzyme_thermodynamics/enzyme_data1.pl?T1=57WOL/BAL_1173 57WOL/BAL_1173]]) | ||
| + | |- | ||
| + | | 3.92 | ||
| + | | pH=7, T=298.15 K, c(MgSO4,mol dm-3) =0.01 | ||
| + | | From Wold et al. (1957) (NIST database<ref name="nist"></ref> [[http://xpdb.nist.gov/enzyme_thermodynamics/enzyme_data1.pl?T1=57WOL/BAL_1173 57WOL/BAL_1173]]) | ||
|} | |} | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 15:25, 24 June 2014
Enolase, also known as phosphopyruvate hydratase, catalysis the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP). This is the penultimate step of glycolysis.
Contents
Chemical equation
Rate equation
Mono-substrate reversible Michaelis-Menten equation is used. [1]
![\frac{V_{mf}\frac{[2PG]}{K_{2PG}}-V_{mr}\frac{[PEP]}{K_{PEP}}}{1 + \frac{[2PG]}{K_{2PG}} + \frac{[PEP]}{K_{PEP}}}](/wiki/images/math/6/a/9/6a9ca37a2fd76b4eb56a1e6a5cfb67d0.png)
Modified rate law to take Thermodynamic constraint into consideration
![\frac{V_{mf}\frac{[2PG]}{K_{2PG}} \left( 1 -\frac{[PEP]}{K_{eq} [2PG]} \right)}{1 + \frac{[2PG]}{K_{2PG}} + \frac{[PEP]}{K_{PEP}}}](/wiki/images/math/b/3/3/b33ffe063d57a3aa7d01ca57ebd5e7dc.png)
Parameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
0.34 [2] | 
|
HeLa cell line | |

|
0.38[1] | 
| ||
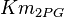
|
0.038[1] | mM | ||
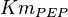
|
0.06[1] | mM |
Parameters with uncertainty
- Three values for
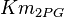 is collected. The values are 0.20 [3], 0.199 [3], 0.038 [1]. The mean and std. dev. is
is collected. The values are 0.20 [3], 0.199 [3], 0.038 [1]. The mean and std. dev. is 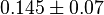
- Similarly for
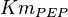 three reported values are 0.58, 0.702, 0.06. The uncertainty is then
three reported values are 0.58, 0.702, 0.06. The uncertainty is then 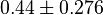 .
.
- In Pietkiewicz et. al. (2009) [3]
 is reported as 1.4
is reported as 1.4 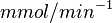 and Marín-Hernández et. al. (2011) [1] reported it to be 0.4. The mean and the std. dev. calculated from these two values are
and Marín-Hernández et. al. (2011) [1] reported it to be 0.4. The mean and the std. dev. calculated from these two values are 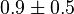 .
.
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
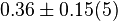 [2] [2]
|

|
HeLa cell line | |

|
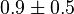
|

| ||
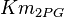
|
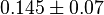
|
mM | Human muscle | |
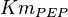
|
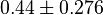
|
mM | Human muscle |
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 3.6 | pH=7, T=25°C | Voet et al.[4] from Newshole et al. (1973) [5]p 97:
|
| 6.7 | T=25°C | Bergmeyer Methods of enzymatic analysis page 449[6] |
| 2.03 | pH=7, T=297.15 K | From Meyerhof et al. (1947)[7]:
|
| 4.29 | pH=7, T=298.15 K, c(MgSO4,mol dm-3) =0.001 | From Wold et al. (1957) (NIST database[8] [57WOL/BAL_1173]) |
| 3.92 | pH=7, T=298.15 K, c(MgSO4,mol dm-3) =0.01 | From Wold et al. (1957) (NIST database[8] [57WOL/BAL_1173]) |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ 2.0 2.1 Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)
- ↑ 3.0 3.1 3.2 Pietkiewicz, J., Gamian, A., Staniszewska, M., & Danielewicz, R. (2009), Inhibition of human muscle-specific enolase by methylglyoxal and irreversible formation of advanced glycation end products, Journal of Enzyme Inhibition and Medicinal Chemistry, 24, 356–364
- ↑ Voet, D., Voet., J.G. and Pratt, C. W. (1999) Fundamentals of biochemistry, Wiley
- ↑ Newshole, E.A. and Stuart, C. (1973) Regulation in Metabolism, Wiley
- ↑ Bergmeyer H.U. (1974) Methods of enzymatic analysis, Publisher: Verlag Chemie (vol 1)
- ↑ Meyerhof O. and Oesper P. (1947) J. Biol. Chem. 170(1):1-22 [J. Biol. Chem.]
- ↑ 8.0 8.1 Goldberg R.N., Tewari Y.B. and Bhat T.N. (2004) Bioinformatics 20(16):2874-2877 [pmid: 15145806]
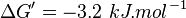 ,
, 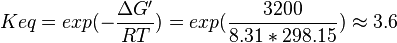
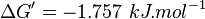 ,
,