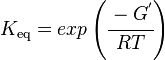Difference between revisions of "Double-bond reductase (DBR)"
Aliah.hawari (talk | contribs) (→What we know) |
Aliah.hawari (talk | contribs) (→Enzyme and Metabolite Background Information) |
||
| Line 59: | Line 59: | ||
! style="border: 1px solid black; padding: 5px; background: #ADD8E6;"|PlantCyc | ! style="border: 1px solid black; padding: 5px; background: #ADD8E6;"|PlantCyc | ||
|- | |- | ||
| − | | | + | | pulegone reductase |
| − | | | + | | PGR |
| | | | ||
| − | | | + | | 37914 Da |
| | | | ||
| | | | ||
| | | | ||
| − | | 1. | + | | 1.3.1.81 |
| − | | | + | | |
|- | |- | ||
| − | | | + | | pulegone |
| − | | | + | | |
| − | | C<sub>10</sub>H<sub>16</sub> | + | | C<sub>10</sub>H<sub>16</sub>O |
| 136.24 | | 136.24 | ||
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| | | | ||
| | | | ||
|- | |- | ||
| − | | | + | | menthone |
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| | | | ||
| − | | | + | | |
| | | | ||
| | | | ||
| Line 109: | Line 109: | ||
| | | | ||
|- | |- | ||
| − | | | + | |isomenthone |
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| | | | ||
| − | | | + | | |
| − | | | + | | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| | | | ||
| | | | ||
| | | | ||
| | | | ||
| + | |- | ||
|} | |} | ||
Revision as of 15:56, 25 May 2016
You can go back to main page of the kinetic model here.
Legend:
| Have not started ·· | 1 -2 data found ·· | 3-4 data found ·· | sufficient data found/estimated ·· | data distribution generated ·· | data sampled |
| to do | DONE! |
Contents
What we know
Pulegone reductase(s) (PGR) catalyses the NADPH-dependent convertion of pulegone to menthone and isomenthone (the former predominates).
Issues
Strategies
Reaction catalysed
Enzyme and Metabolite Background Information
Long metabolite names are abbreviated in the model for clarity and standard identification purposes.
| Metabolite | Abbreviation | Chemical Formula | Molar mass (g/mol) | ChEBI | ChEMBL | PubChem | BRENDA | PlantCyc |
|---|---|---|---|---|---|---|---|---|
| pulegone reductase | PGR | 37914 Da | 1.3.1.81 | |||||
| pulegone | C10H16O | 136.24 | ||||||
| menthone | ||||||||
| NADPH | C21H30N7O17P3 | 745.42116 | 16474 | |||||
| NADP+ | C21H29N7O17P3 | 744.41322 | 18009 | |||||
| isomenthone |
Equation Rate
Limonene-hydroxylase (L3H) is modelled using the reversible Michaelis-Menten equation.
| Parameter | Description | Reference |
|---|---|---|
| VL3H | Reaction rate for Limonene-3-hydroxylase | ref |
| Vmaxforward | Maximum reaction rate towards the production of (-)-trans-isopiperitenol | ref |
| Kmlimonene | Michaelis-Menten constant for Limonene | ref |
| Kmisopiperitenol | Michaelis-Menten constant for (-)-trans-isopiperitenol | ref |
| KmNADPH | Michaelis-Menten constant for NADPH | ref |
| KmNADP | Michaelis-Menten constant for NADP+ | ref |
| Keq | Equilibrium constant | ref |
| [limonene] | Limonene concentration | ref |
| [isopiperitenol] | (-)-trans-isopiperitenol concentration | ref |
| [NADPH] | NADPH concentration | ref |
| [NADP] | NADP+ concentration | ref |
Strategies for estimating the kinetic parameter values
Standard Gibbs Free energy
The Gibbs free energy for PGR is -3.9565125 kcal.mol^-1. This value is estimated from the 'Contribution group' method by Latendresse, M. and is available from MetaCyc (EC 1.3.1.81) [1].
Calculating the Equilibrium Constant
The equilibrium constant can be calculated using the Van't Hoff Isotherm equation:
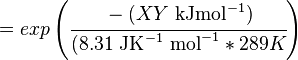
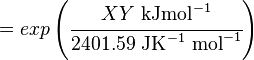
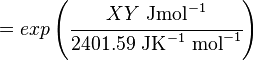
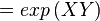
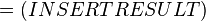
where;
| Keq | Equilibrium constant |
| -ΔG° | Gibbs free energy change. For (INSERT ENZYME) it is (INSERT VALUE) kJmol-1 |
| R | Gas constant with a value of 8.31 JK-1mol-1 |
| T | Temperature which is always expressed in kelvin |
Extracting Information from (INSERT SUBSTRATE/PRODUCT) Production Rates
A table will go here
Published Kinetic Parameter Values
A table will go here.
Detailed description of kinetic values obtained from literature
A more detailed description of the values listed above can be found here .
Simulations
References
- ↑ Latendresse M. (2013). "Computing Gibbs Free Energy of Compounds and Reactions in MetaCyc."
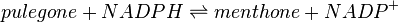
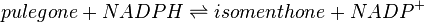
![V_\mathrm{L3H} = Vmax_\mathrm{forward} * \cfrac {\left ( \cfrac{[limonene]}{Km_\mathrm{limonene}} * \cfrac {[NADPH]}{Km_\mathrm{NADPH}} \right ) * \left ( 1 - \cfrac {[isopiperitenol]*[NADP]}{[limonene]*[NADPH]*K_\mathrm{eq}} \right )}
{ \left (1 + \cfrac {[NADPH]}{Km_\mathrm{NADPH}} + \cfrac {[NADP]}{Km_\mathrm{NADP}} \right ) + \left ( 1+ \cfrac {[limonene]}{Km_\mathrm{limonene}} + \cfrac {[isopiperitenol]}{Km_\mathrm{isopiperitenol}} \right ) }](/wiki/images/math/6/3/7/6372d5716522f3a6100b47a63f36e873.png)