Difference between revisions of "Diffusion of C and Ce"
(→Rate equation) |
(→Rate equation) |
||
| Line 11: | Line 11: | ||
== Rate equation == | == Rate equation == | ||
| − | The rate equation is different for the two reactions in order to express the dynamics of the cell density in the environment. Therefore, an additional term <math>r</math> is added in the rate of diffusion from the external environment to the cell. | + | The rate equation is different for the two reactions in order to express the dynamics of the cell density in the environment. Therefore, an additional term <math>r= V_{cell,tot}/V_{ext}</math> is added in the rate of diffusion from the external environment to the cell. This term represents the additional propensity given to the diffusion of extracellular SCB1 by the accumulation of cells in the environment and the consequent increase of cell density. The two rate laws are therefore formed as follows: |
| − | <center><math> | + | <center><math> r_{out}= D \cdot ([C_{e}]-[C])</math></center> |
| − | <center><math> | + | <center><math> r_{in}= r \cdot D \cdot([C]-[C_{e}])</math></center> |
== Parameters == | == Parameters == | ||
Revision as of 01:31, 29 September 2015
The SCB1 protein (C) diffuses from each cell to the environment and back.
Contents
Chemical equation
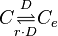
Rate equation
The rate equation is different for the two reactions in order to express the dynamics of the cell density in the environment. Therefore, an additional term 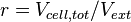 is added in the rate of diffusion from the external environment to the cell. This term represents the additional propensity given to the diffusion of extracellular SCB1 by the accumulation of cells in the environment and the consequent increase of cell density. The two rate laws are therefore formed as follows:
is added in the rate of diffusion from the external environment to the cell. This term represents the additional propensity given to the diffusion of extracellular SCB1 by the accumulation of cells in the environment and the consequent increase of cell density. The two rate laws are therefore formed as follows:
![r_{out}= D \cdot ([C_{e}]-[C])](/wiki/images/math/b/e/1/be1bfbe3de85b2144bfc73b5cd16a5c2.png)
![r_{in}= r \cdot D \cdot([C]-[C_{e}])](/wiki/images/math/8/4/d/84d9faddb66659ee7bad3500af5bf074.png)
Parameters
The parameter of this reaction is the degradation rate of C ( ).
).
| Name | Value | Units | Origin | Remarks |
|---|---|---|---|---|
|
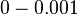 [1] [1]
|

|
Degradation rate of acyl-homoserine lactone (AHL) | The assumption that the degradation of SCB1 is slower than
AHL is made, as described in Takano(2006)[2] |
Parameters with uncertainty
The most plausible parameter value for the 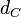 is decided to be
is decided to be 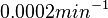 and the confidence interval
and the confidence interval  . This means that the mode of the PDF is 0.0002 and the range where 95% of the values are found is between
. This means that the mode of the PDF is 0.0002 and the range where 95% of the values are found is between 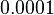 and
and 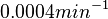 .
.
The probability distribution for the parameter, adjusted accordingly in order to reflect the above values, is the following:
The location and scale parameters of the distribution are:
| Parameter | μ | σ |
|---|---|---|
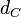
|
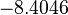
|
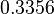
|
References
- ↑ S. Basu, Y. Gerchman, C. H. Collins, F. H. Arnold & R. Weiss. A synthetic multicellular system for programmed pattern formation. Nature 434, 1130-1134, 2005
- ↑ E. Takano. γ-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Current Opinion in Microbiology, 9(3):287–294, 2006.

