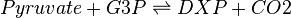DXS
You can go back to main page of the kinetic model here.
Contents
The DXS reaction (EC 2.2.1.7)
Deoxyxylulose-5-phosphate synthase (DXS) catalyses the production of 1-deoxy-D-xylulose 5-phosphate (DXP) from pyruvate and glyceraldehyde 3-phosphate (G3P). This reaction is the first step in the MEP pathway.
Modelling DXS
In the kinetic model, the DXS reaction is modelled with reversible Michaelis-Menten using the Hanekom [1] bi-bi random order generic equation. In total, this reaction requires six kinetic parameters and one thermodynamic parameter (Equilibrium constant, Keq).
Failed to parse (Cannot store math image on filesystem.): V_\mathrm{DXS}= Kcat_\mathrm{forward} . [DXS]
V_\mathrm{GPPS} = Vmax_\mathrm{forward} * \cfrac { \left (\cfrac{[DMAPP]}{Km_\mathrm{DMAPP}} * \cfrac{[IPP]}{Km_\mathrm{IPP}}\right )* \left ( 1 - \cfrac {[GPP]*[PP]}{[DMAPP]*[IPP]*K_\mathrm{eq}} \right )}{\left (1 + \cfrac {[IPP]}{Km_\mathrm{IPP}} + \cfrac {[PP]}{Km_\mathrm{PP}} \right ) * \left ( 1 + \cfrac {[DMAPP]}{Km_\mathrm{DMAPP}} + \cfrac {[GPP]}{Km_\mathrm{GPP}} \right )}
</math>
References
</references>
Enzyme and Metabolite Background Information
- ↑ Hanekom2016