Binding of R2 to C
SCBs (C2) bind to ScbR homo-dimer (R2) and inactivate its repressing activity.
Contents
Chemical equation
The exact mechanism is still unclear, however in our model we assumed that two molecules of SCB1 bind to the ScbR homo-dimer.
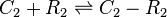
Rate equation
![r= \frac{k^{-}_{4}}{K_{d4}}\cdot [C_{2}]\cdot [R_{2}] - k^{-}_{4}\cdot [C_{2}-R_{2}]](/wiki/images/math/8/b/1/8b1532da3ed7157bf938225feb25d681.png)
Parameters
The parameters of this reaction are the dissociation constant for binding of SCB1 to ScbR (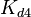 ) and the dissociation rate for binding of SCB1 to ScbR (
) and the dissociation rate for binding of SCB1 to ScbR (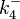 ).
).
| Name | Value | Units | Origin | Remarks |
|---|---|---|---|---|
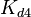
|
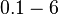 [1] [2] [3] [4] [1] [2] [3] [4]
|
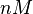
|
TetR-like Rv3066 from M. tuberculosis
TetR in complex with the inducer tetracycline-Mg2+ |
Repressor protein TetR binds to [MgTc]+ and its affinity for the operator tetO is 9-fold reduced
Similar structure and activity as Scb1 binding to ScbR. |
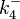
|
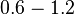 [5] [6] [5] [6]
|

|
SPR of a TetR-like protein (RolR) on a Gram and
GC content ~ 50-60% from Corynebacterium glutamicum |
Parameters with uncertainty
The most plausible parameter value for the 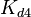 is decided to be
is decided to be 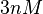 and the confidence interval
and the confidence interval 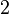 . This means that the mode of the PDF is 3 and the range where 95% of the values are found is between 1.5 and 6 nM.
. This means that the mode of the PDF is 3 and the range where 95% of the values are found is between 1.5 and 6 nM.
In a similar way, the most plausible value for 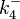 is
is 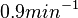 and the confidence interval
and the confidence interval 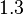 . This means that the mode of the PDF is 0.9 and the range where 95% of the values are found is between 0.6923 and 1.17
. This means that the mode of the PDF is 0.9 and the range where 95% of the values are found is between 0.6923 and 1.17  .
.
The probability distributions for the two parameters, adjusted accordingly in order to reflect the above values, are the following:
The location and scale parameters of the distributions are:
| Parameter | μ | σ |
|---|---|---|
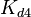
|
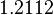
|
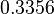
|
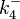
|
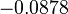
|
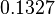
|
References
- ↑ Bolla JR, Do SV, Long F, et al. Structural and functional analysis of the transcriptional regulator Rv3066 of Mycobacterium tuberculosis. Nucleic Acids Research. 2012;40(18):9340-9355.
- ↑ Ahn SK, Tahlan K, Yu Z, Nodwell J. Investigation of Transcription Repression and Small-Molecule Responsiveness by TetR-Like Transcription Factors Using a Heterologous Escherichia coli-Based Assay. Journal of Bacteriology. 2007;189(18):6655-6664.
- ↑ Orth P., Schnappinger D, Hillen W, Saenger W, Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nature Structural & Molecular Biology, 2000;7(3):215-9.
- ↑ E. Takano. γ-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Current Opinion in Microbiology, 9(3):287–294, 2006.
- ↑ Sylwia Kedracka-Krok, Andrzej Gorecki, Piotr Bonarek, and Zygmunt Wasylewski. Kinetic and Thermodynamic Studies of Tet Repressor−Tetracycline Interaction. Biochemistry 2005 44 (3), 1037-1046.
- ↑ Li T, Zhao K, Huang Y, et al. The TetR-Type Transcriptional Repressor RolR from Corynebacterium glutamicum Regulates Resorcinol Catabolism by Binding to a Unique Operator, rolO. Applied and Environmental Microbiology. 2012;78(17):6009-6016.

