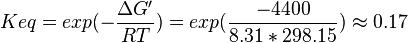3-phosphoglycerate mutase
Phosphoglycerate mutase (PGAM) is an enzyme that catalyzes the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG).
Contents
Chemical equation
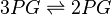
Rate equation
Mono-substrate reversible Michaelis-Menten equation is used. [1]
![\frac{V_{mf}\frac{[3PG]}{Km_{3PG}}-V_{mr}\frac{[2PG]}{Km_{2PG}}}{1 + \frac{[3PG]}{Km_{3PG}} + \frac{[2PG]}{Km_{2PG}}}](/wiki/images/math/6/f/e/6fed036845e1a93d67d5db4525e750af.png)
Modified rate law to take Thermodynamic constraint into consideration
![\frac{V_{mf}\frac{[3PG]}{Km_{3PG}} \left( 1 -\frac{[2PG]}{K_{eq}[3PG]} \right)}{1 + \frac{[3PG]}{Km_{3PG}} + \frac{[2PG]}{Km_{2PG}}}](/wiki/images/math/b/9/9/b991571eae8e39f2d7f38bbe953665f2.png)
Parameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
0.94 [1] | 
|
HeLa cell line | |

|
0.36 [1] | 
| ||
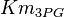
|
0.19[1] | mM | ||
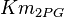
|
0.12[1] | mM |
Parameters with uncertainty
- Two values of
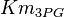 are found for Human cells. They are reported as 0.28[2] and 0.12[1]. Averaging them gives mean value of 0.2 and 0.08
are found for Human cells. They are reported as 0.28[2] and 0.12[1]. Averaging them gives mean value of 0.2 and 0.08
- As the value of the
 does not depend on the organism, the mean and std. dev. of the distribution can be calculated from the various values reported in the literature. [4]
does not depend on the organism, the mean and std. dev. of the distribution can be calculated from the various values reported in the literature. [4]  can be sampled based on Haldane equation
can be sampled based on Haldane equation
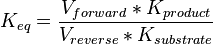 or Alternatively, same percentage of error mentioned for
or Alternatively, same percentage of error mentioned for  considering the value for
considering the value for  reported in the model file of Hernandez (2011)[1]. This gives the value of
reported in the model file of Hernandez (2011)[1]. This gives the value of 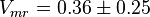
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
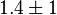 [5] [5]
|

|
HeLa cell line | |

|
Sampled based on Haldane equation 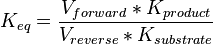
|

| ||
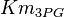
|
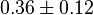
|
mM | Multiple tissue of Human cell | |
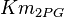
|
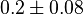
|
mM | Multiple tissue of Human cell |
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 0.15 | pH=7, T=25°C | Voet et al.[6] from Newshole et al. (1973) [7]p 97:
|
| 0.17 | pH=7, T=25°C | Lehninger, (2008)[8] p 553:
|
| 0.167 | pH=7, T=297.15 K | From Meyerhof et al. 1949 (NIST database[9] [49MEY/OES_1388]) |
| 0.19 | pH=7.2, T=25°C | From Chiba et al. 1959 (NIST database[9] [59CHI/SUG_1391]) |
| 0.20 | pH=6, T=25°C | From Grisolia et al. 1975 (NIST database[9] [75GRI/CAR_1396]) |
Averaging all those values gives 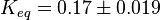
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ 2.0 2.1 Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S. & Moreno-Sanchez, R. (2009), HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini-Rev. Med. Chem. 9, 1084–1101
- ↑ de Atauri, P.; Repiso, A.; Oliva, B.; Vives-Corrons, J.L.; Climent, F.; Carreras, J. (2005), Characterization of the first described mutation of human red blood cell phosphoglycerate mutase, Biochim. Biophys. Acta 1740, 403-410
- ↑ Achcar, F., Kerkhoven, E. J., Bakker, B. M., Barrett, M. P., Breitling, R. (2012), Dynamic modelling under uncertainty: the case of Trypanosoma brucei energy metabolism, PLoS Comput. Biol. 8, e1002352.
- ↑ Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)
- ↑ Voet, D., Voet., J.G. and Pratt, C. W. (1999) Fundamentals of biochemistry, Wiley
- ↑ Newshole, E.A. and Stuart, C. (1973) Regulation in Metabolism, Wiley
- ↑ David L. Nelson, Michael M. Cox (2008), Lehninger Principles of Biochemistry (5th edn), W. H. Freeman and Company
- ↑ 9.0 9.1 9.2 Goldberg R.N., Tewari Y.B. and Bhat T.N. (2004) Bioinformatics 20(16):2874-2877 [pmid: 15145806]
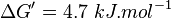 ,
, 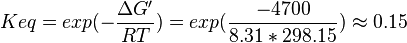
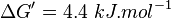 ,
,