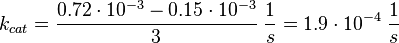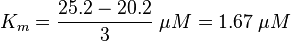Rap1-Activation
Reaction
by EPAC:
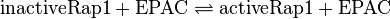
by C3G:
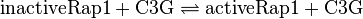
Rate Equation
by EPAC:
![v_{\text{Rap1-Activation}} = \frac{k_{cat} \cdot [\text{EPAC}] \cdot [\text{inactiveRap1}] \cdot (1-\frac{[\text{activeRap1}]}{[\text{inactiveRap1}] \cdot K_{eq}})}{Km_{\text{inactiveRap1}} \cdot (1 + \frac{[\text{inactiveRap1}]}{Km_{\text{inactiveRap1}}} + \frac{[\text{activeRap1}]}{Km_{\text{activeRap1}}})}](/wiki/images/math/6/8/7/68763d385573e2dc888745ddb681801a.png)
by C3G:
![v_{\text{Rap1-Activation}} = \frac{k_{cat} \cdot [\text{C3G}] \cdot [\text{inactiveRap1}] \cdot (1-\frac{[\text{activeRap1}]}{[\text{inactiveRap1}] \cdot K_{eq}})}{Km_{\text{inactiveRap1}} \cdot (1 + \frac{[\text{inactiveRap1}]}{Km_{\text{inactiveRap1}}} + \frac{[\text{activeRap1}]}{Km_{\text{activeRap1}}})}](/wiki/images/math/e/8/5/e85c67b24ac2c60d15e4a75acfe871eb.png)
final Parameter
Activation by EPAC
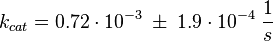
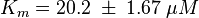
Activation by C3G
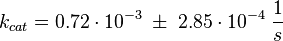
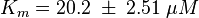
Parameter
| Notes | References |
|---|---|
|
Tsalkova et al. [1] found by mutating phenylalanin at position 435 that this amino acid is the best one for allowing hinge motion by keeping the binding side closed in the absence of cAMP. An exchange of this amino acid to thryptophane leads to a decrease in the enzyme activity. Because it is likely that the true value is in the range of plus/minus the difference between the measured values of the wt enzyme and the mutant the standard deviation is set to a third of this difference, because then 99 % of the values lie in this range. Uncertainty estimation:
The uncertainty for the activation by C3G is larger, because it is not the reaction measured. Therefore the calculated error for the activation by EPAC is increased by 50%. |
 Tsalkova et al. (2009) [1] |
KmP value and equilibrium constant
The Km value for the product is assumed to be similar but slightly higher than the the Km value of the substrate because of the similarity of the both species. Therefore the Km value of the substrate is multiplied by 1.05 to gain the one of the product and the uncertainty is increased by increasing the error on Kmsubstrate by 50%.
For information about the equilibrium constant please see here.
References
- ↑ 1.0 1.1 Tsalkova T. et al (2009) Mechanism of EPAC activation; Structural and functional analyses of Epac2 hinge mutants with constitutive and reduced activities J.Biol.Chem. 284:23644-23651 DOI: 10.1074/jbc.M109.024950. (pmid:19553663)