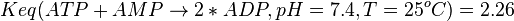Adenylate kinase
Adenylate kinase is a phosphotransferase enzyme that catalyzes the interconversion of adenine nucleotides.
Contents
Chemical equation
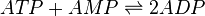
Rate equation
Reversible mass action rate law is used
![K_{1}[ATP][AMP] - K_{2}[ADP]^2](/wiki/images/math/7/f/c/7fc054b0299ef7cf1db75a18a2860588.png)
Parameter values
| Parameter | Value | Organism | Remarks |
|---|---|---|---|
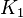
|
1 [1] | HeLa cell line | |
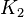
|
2.26 [1] |
Parameters with uncertainty
| k1=442, k2=1000 | The kinetic parameters of adenylate kinase are unknown. Therefore, it was modeled using mass action kinetics with parameters k1 and k2 consistent with the equilibrium constant of the reaction. The equilibrium constant (Keq=0.442) is from Bergmeyer H.U. (1974) page 486[2]:
|
Equilibrium constant
| Equilibrium constant | Conditions | Source |
|---|---|---|
| 0.48+/-0.015 (mean+/-SEM; n=7) | pH=7, T=25°C, 10mM Mg2+ | NIST database "Thermodynamics of Enzyme-Catalyzed Reactions" entry [61ATK/BUR_640] from Atkinson et al. (1961) [3] Table 2: Therefore, Keq(forward) = 0.48 +/-0.015 (n=7; mean+/-SEM calculated from individual measurements). |
References
- ↑ 1.0 1.1 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ Bergmeyer H.U. (1974) Methods of enzymatic analysis, Publisher: Verlag Chemie (vol 1)
- ↑ Atkinson, M. R., Burton, R. M. and Morton, R. K. (1961) Biochem J. 78(4):813–820. (pmid: 13684980)