Lactate dehydrogenase
A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. Lactate dehydrogenase catalyzes the conversion of pyruvate to lactate and back, as it converts NADH to NAD+ and back.
Contents
Chemical reactions
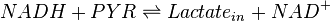
Rate equation
RAndom Bi-Bi reversible Michaelis-Menten equation is used. [1]
![\frac{V_{mf}\frac{[NADH][PYR]}{Km_{NADH} K_{PYR}} - V_{mr}\frac{[Lactate_{in}][NAD]}{Km_{Lactate_{in}} K_{NAD}}}{1 + \frac{[NADH]}{Km_{NADH}} + \frac{[PYR]}{Km_{PYR}} + \frac{[NADH][PYR]}{Km_{NADH} Km_{PYR}} + \frac{[Lactate_{in}][NAD]}{Km_{Lactate_{in}} Km_{NAD}} + \frac{[Lactate_{in}]}{Km_{Lactate_{in}}} + \frac{[PYR]}{Km_{PYR}} }](/wiki/images/math/4/b/2/4b2b29cf3850c4c61d11218132ecd486.png)
Prameter values
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
3.4 [1] | 
|
HeLa cell line | |

|
0.54 | 
|
HeLa cell line | |
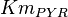
|
0.1 | mM | HeLa cell line | |
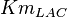
|
4.7 | mM | Rat AS-30D hepatoma | |
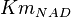
|
0.07 | mM | HeLa cell line | |
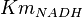
|
0.002 | mM | HeLa cell line |
Parameters with uncertainty
- Mean and Std. Dev. for
 has been reported in Table S3 for Marín-Hernández (2011) et. al. [1]. The Std. Dev. for
has been reported in Table S3 for Marín-Hernández (2011) et. al. [1]. The Std. Dev. for  is calculated based on the same ratio for
is calculated based on the same ratio for  .
.
| Parameter | Value | Units | Organism | Remarks |
|---|---|---|---|---|

|
Failed to parse (Cannot store math image on filesystem.): 3.4 \pm 0.5 (3) [2] | 
|
HeLa cell line | |

|
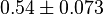
|

|
HeLa cell line | |
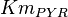
|
0.1 | mM | HeLa cell line | |
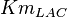
|
4.7 | mM | Rat AS-30D hepatoma | |
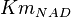
|
0.07 | mM | HeLa cell line | |
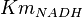
|
0.002 | mM | HeLa cell line |
References
- ↑ 1.0 1.1 1.2 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)