Dephosphorylation
Binding Reaction
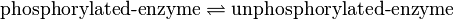
Kinetic equation
![\frac{v_{max} \cdot [\text{phosphorylated-enzyme}] \cdot (1-\frac{[\text{unphosphorylated-enzyme}]}{[\text{phosphorylated-enzyme}] \cdot K_{eq}})}{1 + \frac{[\text{phosphorylated-enzyme}]}{Km_{\text{phosphorylated-enzyme}}} \cdot \frac{[\text{unphosphorylated-enzyme}]}{Km_{\text{unphosphorylated-enzyme}}}}](/wiki/images/math/7/1/9/7198fa49027c81ad9176a192bf39e233.png)
final Parameter
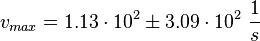
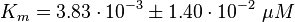
Parameter
| Notes | Data | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| There are three main deophosphatases, phosphoserine phosphatase (EC 3.1.3.3), phosphoprotein phosphatase (EC 3.1.3.16), protein-tyrosine phosphatase (3.1.3.48). The data available in BRENDA is used to calculate the average and standard deviation. |
|