Difference between revisions of "3-phosphoglycerate kinase"
| Line 8: | Line 8: | ||
== Rate equation == | == Rate equation == | ||
| − | Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used. | + | Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used. <ref name="Hernandez2011"> Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 ([http://dx.doi.org/10.1016/j.bbabio.2010.11.006 doi]) </ref> |
| + | <center><math> \frac{V_{mf}\frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} - V_{mr}\frac{[3PG][ATP]}{K_{3PG} K_{ATP}}}{1 + \frac{[1,3BPG]}{K_{1,3BPG}} + \frac{[ADP]}{K_{ADP}} + \frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} + \frac{[3PG][ATP]}{K_{3PG} K_{ATP}} + \frac{[3PG]}{K_{3PG}} + \frac{[ADP]}{K_{ADP}} } </math></center> | ||
| + | == Parameter values == | ||
| − | + | {|class="wikitable" | |
| + | ! Parameter | ||
| + | ! Value | ||
| + | ! Organism | ||
| + | ! Remarks | ||
| + | |- | ||
| + | |<math>V_{mf}</math> | ||
| + | |13 <ref name="Hernandez_2006"> Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, ''et al.'' (2006). ''Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase''. FEBS J., 273 , pp. 1975–1988([http://dx.doi.org/doi:10.1111/j.1742-4658.2006.05214.x doi]) </ref> | ||
| + | |rowspan="6"|HeLa cell line | ||
| + | |rowspan="6"| | ||
| + | |- | ||
| + | |<math>V_{mr}</math> | ||
| + | |3.8<ref name="Hernandez2011"></ref> | ||
| + | |- | ||
| + | |<math>Km_{1,3BPG}</math> | ||
| + | |0.079<ref name="Hernandez2011"></ref> | ||
| + | |- | ||
| + | |<math>Km_{3PG}</math> | ||
| + | |0.13<ref name="Hernandez2011"></ref> | ||
| + | |- | ||
| + | |<math>Km_{ADP}</math> | ||
| + | |0.04<ref name="Hernandez2011"></ref> | ||
| + | |- | ||
| + | |<math>Km_{ATP}</math> | ||
| + | |0.27<ref name="Hernandez2011"></ref> | ||
| + | |} | ||
| − | |||
| − | |||
== Alternative parameter values == | == Alternative parameter values == | ||
Revision as of 16:01, 4 March 2014
Phosphoglycerate kinase (PGK) is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP. Like all kinases it is a transferase.
Contents
Chemical equation
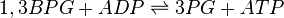
Rate equation
Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used. [1]
![\frac{V_{mf}\frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} - V_{mr}\frac{[3PG][ATP]}{K_{3PG} K_{ATP}}}{1 + \frac{[1,3BPG]}{K_{1,3BPG}} + \frac{[ADP]}{K_{ADP}} + \frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} + \frac{[3PG][ATP]}{K_{3PG} K_{ATP}} + \frac{[3PG]}{K_{3PG}} + \frac{[ADP]}{K_{ADP}} }](/wiki/images/math/8/e/b/8eb366ea68004741a7ace7e2cf32d509.png)
Parameter values
| Parameter | Value | Organism | Remarks |
|---|---|---|---|

|
13 [2] | HeLa cell line | |

|
3.8[1] | ||
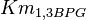
|
0.079[1] | ||
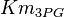
|
0.13[1] | ||
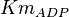
|
0.04[1] | ||
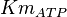
|
0.27[1] |
Alternative parameter values
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Marín-Hernández A, Gallardo-Pérez JC, Rodríguez-Enríquez S et al (2011) Modeling cancer glycolysis. Biochim Biophys Acta 1807:755–767 (doi)
- ↑ Marín-Hernández A , Rodríguez-Enríquez S, Vital-González P A, et al. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J., 273 , pp. 1975–1988(doi)