Difference between revisions of "Phosphohydroxypyruvate"
| Line 5: | Line 5: | ||
==Rate equation== | ==Rate equation== | ||
| − | + | Special rate law is used. <ref name = "Smallbone_2013">Smallbone K, Stanford NJ (2013). ''Kinetic modeling of metabolic pathways: Application to serine biosynthesis''. In: Systems Metabolic Engineering, Humana Press. pp. 113–121</ref> | |
<center><math> \frac{[serB]K_{cat}B \frac{[PSER]}{Kb_{PSER}} }{ 1 + \frac{[PSER]}{Kb_{PSER}} + \frac{[Serine]}{Kb_{Serine}} } </math></center> | <center><math> \frac{[serB]K_{cat}B \frac{[PSER]}{Kb_{PSER}} }{ 1 + \frac{[PSER]}{Kb_{PSER}} + \frac{[Serine]}{Kb_{Serine}} } </math></center> | ||
| Line 12: | Line 12: | ||
==References== | ==References== | ||
| + | <references/> | ||
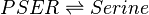
![\frac{[serB]K_{cat}B \frac{[PSER]}{Kb_{PSER}} }{ 1 + \frac{[PSER]}{Kb_{PSER}} + \frac{[Serine]}{Kb_{Serine}} }](/wiki/images/math/9/f/b/9fbf815be62191827028039d32d2d94f.png)