Difference between revisions of "Glycogen synthase"
| Line 4: | Line 4: | ||
MWC model (Monod-Wyman-Changeux model) is used to model the reaction mechanism of this enzyme. | MWC model (Monod-Wyman-Changeux model) is used to model the reaction mechanism of this enzyme. | ||
<center><math> UDPG + Glucose \rightarrow UDP + Glycogen </math></center> | <center><math> UDPG + Glucose \rightarrow UDP + Glycogen </math></center> | ||
| + | |||
| + | ==Rate equation== | ||
| + | <center><math>\frac{K_{cat,r}[GS]n\frac{[UDPG]}{K_{UDPG}} \left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[ATP]}{K'_{r,ATP}} \right)^{n-1}}{\left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[ATP]}{K'_{ATP}} \right)^n L_{0} \left( \frac{1 + \frac{[Glc6P]}{K_{t,Glc6P}} + \frac{[ATP]}{K_{t,ATP}} }{1 + \frac{[Glc6P]}{K_{r,Glc6P}} + \frac{[ATP]}{K_{r,ATP}} } \right)^n + \left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[ATP]}{K'_{r,ATP}} \right)^n }</math></center> | ||
Revision as of 17:29, 28 February 2014
Glycogen synthase (UDP-glucose-glycogen glucosyltransferase) converts glucose to glycogen. It takes short polymers of glucose and converts them into long polymers one by one into a polymeric chain for storage as glycogen.
Chemical equation
MWC model (Monod-Wyman-Changeux model) is used to model the reaction mechanism of this enzyme.
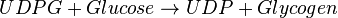
Rate equation
![\frac{K_{cat,r}[GS]n\frac{[UDPG]}{K_{UDPG}} \left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[ATP]}{K'_{r,ATP}} \right)^{n-1}}{\left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[ATP]}{K'_{ATP}} \right)^n L_{0} \left( \frac{1 + \frac{[Glc6P]}{K_{t,Glc6P}} + \frac{[ATP]}{K_{t,ATP}} }{1 + \frac{[Glc6P]}{K_{r,Glc6P}} + \frac{[ATP]}{K_{r,ATP}} } \right)^n + \left( 1 + \frac{[UDPG]}{K_{UDPG}} + \frac{[ATP]}{K'_{r,ATP}} \right)^n }](/wiki/images/math/9/9/d/99de2ee8cfcb0a5850c32783c00922f0.png)