Difference between revisions of "Phosphoglucomutase"
| Line 5: | Line 5: | ||
==Rate equation== | ==Rate equation== | ||
| + | Mono substrate reversible Michelis-Menten with Haldane substitution is used as a rate law. | ||
<center><math> \frac{\frac{V_{max}}{K_{Glc6P}}\left( [Glc6P] - \frac{[Glc1P]}{K_{eq}} \right) }{1 + \frac{[Glc6P]}{K_{Glc6P}} + \frac{[Glc1P]}{K_{Glc1P}} } </math></center> | <center><math> \frac{\frac{V_{max}}{K_{Glc6P}}\left( [Glc6P] - \frac{[Glc1P]}{K_{eq}} \right) }{1 + \frac{[Glc6P]}{K_{Glc6P}} + \frac{[Glc1P]}{K_{Glc1P}} } </math></center> | ||
Revision as of 15:54, 28 February 2014
Phosphoglucomutase (PGLM) facilitates the interconversion of glucose 1-phosphate and glucose 6-phosphate by transfering a phosphate group on an α-D-glucose monomer from the 1' to the 6' position in the forward direction or the 6' to the 1' position in the reverse direction.
Chemical reaction
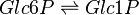
Rate equation
Mono substrate reversible Michelis-Menten with Haldane substitution is used as a rate law.
![\frac{\frac{V_{max}}{K_{Glc6P}}\left( [Glc6P] - \frac{[Glc1P]}{K_{eq}} \right) }{1 + \frac{[Glc6P]}{K_{Glc6P}} + \frac{[Glc1P]}{K_{Glc1P}} }](/wiki/images/math/b/3/b/b3b31230b8a8021f39eedd96660f53ae.png)