Difference between revisions of "3-phosphoglycerate kinase"
| Line 8: | Line 8: | ||
== Rate equation == | == Rate equation == | ||
| − | Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used <ref name="pmid6689547">{{cite journal | author = Yoshida A, Tani K | title = Phosphoglycerate kinase abnormalities: functional, structural and genomic aspects | journal = Biomed. Biochim. Acta | volume = 42 | issue = 11-12 | pages = S263–7 | year = 1983 | pmid = 6689547 | doi = }}</ref> | + | Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used.<ref name="pmid6689547">{{cite journal | author = Yoshida A, Tani K | title = Phosphoglycerate kinase abnormalities: functional, structural and genomic aspects | journal = Biomed. Biochim. Acta | volume = 42 | issue = 11-12 | pages = S263–7 | year = 1983 | pmid = 6689547 | doi = }}</ref> Since |
| Line 19: | Line 19: | ||
== References == | == References == | ||
| − | + | {{reflist}} | |
Revision as of 11:57, 27 February 2014
Phosphoglycerate kinase (PGK) is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP. Like all kinases it is a transferase.
Contents
Chemical equation
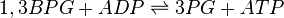
Rate equation
Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used.[1] Since
![\frac{V_{mf}\frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} - V_{mr}\frac{[3PG][ATP]}{K_{3PG} K_{ATP}}}{1 + \frac{[1,3BPG]}{K_{1,3BPG}} + \frac{[ADP]}{K_{ADP}} + \frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} + \frac{[3PG][ATP]}{K_{3PG} K_{ATP}} + \frac{[3PG]}{K_{3PG}} + \frac{[ADP]}{K_{ADP}} }](/wiki/images/math/8/e/b/8eb366ea68004741a7ace7e2cf32d509.png)