Difference between revisions of "3-phosphoglycerate kinase"
| Line 43: | Line 43: | ||
number = {6} | number = {6} | ||
} | } | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
</bibtex> | </bibtex> | ||
Revision as of 11:37, 27 February 2014
Phosphoglycerate kinase (PGK) is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP. Like all kinases it is a transferase.
Chemical equation
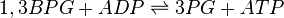
Rate equation
Random Bi-Bi reversible Michaelis-Menten euation for non-interacting substrates are used .
![\frac{V_{mf}\frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} - V_{mr}\frac{[3PG][ATP]}{K_{3PG} K_{ATP}}}{1 + \frac{[1,3BPG]}{K_{1,3BPG}} + \frac{[ADP]}{K_{ADP}} + \frac{[1,3BPG][ADP]}{K_{1,3BPG} K_{ADP}} + \frac{[3PG][ATP]}{K_{3PG} K_{ATP}} + \frac{[3PG]}{K_{3PG}} + \frac{[ADP]}{K_{ADP}} }](/wiki/images/math/8/e/b/8eb366ea68004741a7ace7e2cf32d509.png)
Parameter values
Alternative parameter values
Vrizlynn L.L. Thing, Tong-Wei Chua, Ming-Lee Cheong - Modeling cancer glycolysis
- Biochimica et Biophysica Acta (BBA) - Bioenergetics 1807(6):755 - 767, Los Alamitos, CA, USA, 11 2011
- BibtexAuthor : Vrizlynn L.L. Thing, Tong-Wei Chua, Ming-Lee Cheong
Title : Modeling cancer glycolysis
In : Biochimica et Biophysica Acta (BBA) - Bioenergetics -
Address : Los Alamitos, CA, USA
Date : 11 2011