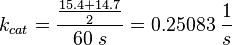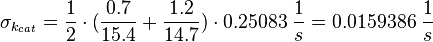Difference between revisions of "MEK-Activation(Raf1)"
m |
m |
||
| Line 25: | Line 25: | ||
<!--First the measured values are divided by the enzyme amount used. In the paper it says: "The assay mixture contains either 0.82 nM COT30-397, [...] in the presence of 5 mM MnCl<sub>2</sub>; or 1.7 nM COT30-397,[...] in the presence of 10 mM MgCl<sub>2</sub>."<ref> Yong Jia et al. (2005) "Purification and kinetic characterization of recombinant human mitogen-activated protein kinase kinase kinase COT and the complexes with its cellular partner NF-kappa B1 p105." ''Arch Biochem Biophys'' 441.1, pp. 64-74. ([http://www.ncbi.nlm.nih.gov/pubmed/16087150 pmid:16087150]) </ref> | <!--First the measured values are divided by the enzyme amount used. In the paper it says: "The assay mixture contains either 0.82 nM COT30-397, [...] in the presence of 5 mM MnCl<sub>2</sub>; or 1.7 nM COT30-397,[...] in the presence of 10 mM MgCl<sub>2</sub>."<ref> Yong Jia et al. (2005) "Purification and kinetic characterization of recombinant human mitogen-activated protein kinase kinase kinase COT and the complexes with its cellular partner NF-kappa B1 p105." ''Arch Biochem Biophys'' 441.1, pp. 64-74. ([http://www.ncbi.nlm.nih.gov/pubmed/16087150 pmid:16087150]) </ref> | ||
<math>\frac{15.4\; \frac{1}{min}}{8.2 \cdot 10^{-4}\; \mu M}\; \pm\; \frac{0.7\; \frac{1}{min}}{8.2 \cdot 10^{-4}\; \mu M} = 1.8780 \cdot 10^{4}\; \pm\; 8.5366 \cdot 10^{2}\; \frac{1}{\mu M \cdot min}</math> | <math>\frac{15.4\; \frac{1}{min}}{8.2 \cdot 10^{-4}\; \mu M}\; \pm\; \frac{0.7\; \frac{1}{min}}{8.2 \cdot 10^{-4}\; \mu M} = 1.8780 \cdot 10^{4}\; \pm\; 8.5366 \cdot 10^{2}\; \frac{1}{\mu M \cdot min}</math> | ||
| − | |||
| − | |||
<math>\frac{14.7\ \frac{1}{min}}{1.7 \cdot 10^{-3}\; \mu M}\; \pm\; \frac{1.2\ \frac{1}{min}}{1.7 \cdot 10^{-3}\; \mu M} = 8.6471 \cdot 10^{3}\; \pm\; 7.0588 \cdot 10^{2}\; \frac{1}{\mu M \cdot min}</math>--> | <math>\frac{14.7\ \frac{1}{min}}{1.7 \cdot 10^{-3}\; \mu M}\; \pm\; \frac{1.2\ \frac{1}{min}}{1.7 \cdot 10^{-3}\; \mu M} = 8.6471 \cdot 10^{3}\; \pm\; 7.0588 \cdot 10^{2}\; \frac{1}{\mu M \cdot min}</math>--> | ||
| − | |||
The values are averaged and then divided by 60 s to change the unit. | The values are averaged and then divided by 60 s to change the unit. | ||
| − | <math> k_{cat} = \frac{\frac{15.4 + 14.7}{2}}{60\; s | + | <math> k_{cat} = \frac{\frac{15.4 + 14.7}{2}}{60\; s} = 0.25083\; \frac{1}{s}</math> |
<math>\sigma_{k_{cat}} = \frac{1}{2} \cdot (\frac{0.7}{15.4} + \frac{1.2}{14.7})\cdot 0.25083\; \frac{1}{s} = 0.0159386\; \frac{1}{s}</math> | <math>\sigma_{k_{cat}} = \frac{1}{2} \cdot (\frac{0.7}{15.4} + \frac{1.2}{14.7})\cdot 0.25083\; \frac{1}{s} = 0.0159386\; \frac{1}{s}</math> | ||
Latest revision as of 10:28, 12 June 2014
Binding Reaction
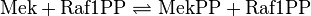
Kinetic Equation
![v_{\text{Mek-Activation}} = \frac{k_{cat} \cdot [\text{Raf1PP}] \cdot [\text{Mek}] \cdot (1-\frac{[\text{MekPP}]}{[\text{Mek}] \cdot K_{eq}})}{Km_{\text{Mek}} \cdot (1 + \frac{[\text{Mek}]}{Km_{\text{Mek}}} + \frac{[\text{MekPP}]}{Km_{\text{MekPP}}})}](/wiki/images/math/f/9/8/f98adc79397926693c59afee56559b1e.png)
final Parameter
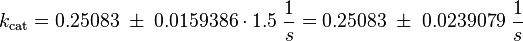
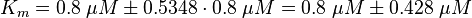
Parameter Reference
| Parameter Calculation | Reference |
|---|---|
|
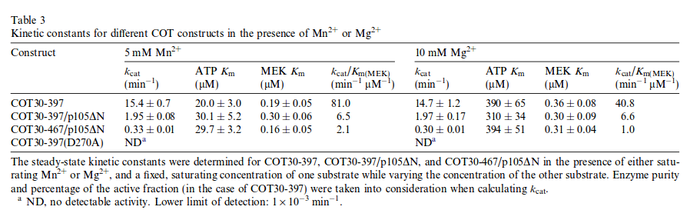
kcat: The values are averaged and then divided by 60 s to change the unit.
Force et al. measured a Km for c-Raf1: For the uncertainty estimation the averaged relative error is used. |
Yong Jia et al. (2005) [2] table 3 |
KmP value and equilibrium constant
The Km value for the product is assumed to be similar but slightly higher than the the Km value of the substrate because of the similarity of the both species. Therefore the Km value of the substrate is multiplied by 1.05 to gain the one of the product and the uncertainty is increased by increasing the error on Kmsubstrate by 50%.
For information about the equilibrium constant please see here.
References
- ↑ Force T. et al (1994) "Enzymatic characteristics of the c-Raf1 protein kinase." Proc Natl Acad Sci USA 91.4:1270-1274
- ↑ Yong Jia et al. (2005) "Purification and kinetic characterization of recombinant human mitogen-activated protein kinase kinase kinase COT and the complexes with its cellular partner NF-kappa B1 p105." Arch Biochem Biophys 441.1, pp. 64-74. (pmid:16087150)