Difference between revisions of "Glyceraldehyde-3-phosphate dehydrogenase"
| Line 2: | Line 2: | ||
== Chemical equation == | == Chemical equation == | ||
| − | <center><math> </math></center> | + | <center><math> NAD + G3P + Pi \rightleftharpoons 1,3BPG + NADH </math></center> |
| + | |||
| + | == Rate equation == | ||
| + | v = \frac{ V_{mf}\frac{[A][B][C]}{K_aK_bK_c} - V_{mr} \frac{[P][Q]}{K_pK_q} }{1 + \frac{[A]}{K_a} + \frac{[A][B]}{K_aK_b} + \frac{[A][B][C]}{K_aK_bK_c} + \frac{[P][Q]}{K_pK_q} + +\frac{[Q]}{K_q} } | ||
Revision as of 18:29, 26 February 2014
This enzyme serves two functions in this step. First the enzyme transfers a hydrogen (H-) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next it adds a phosphate (P) from the cytosol to the oxidized glyceraldehyde phosphate to form 1, 3-bisphosphoglycerate.
Chemical equation
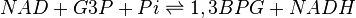
Rate equation
v = \frac{ V_{mf}\frac{[A][B][C]}{K_aK_bK_c} - V_{mr} \frac{[P][Q]}{K_pK_q} }{1 + \frac{[A]}{K_a} + \frac{[A][B]}{K_aK_b} + \frac{[A][B][C]}{K_aK_bK_c} + \frac{[P][Q]}{K_pK_q} + +\frac{[Q]}{K_q} }