Difference between revisions of "Triosephosphate isomerase"
(Created page with "This enzyme rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be use...") |
|||
| Line 7: | Line 7: | ||
Reversible Michaelis-Menten is used | Reversible Michaelis-Menten is used | ||
| − | <center><math> v = \frac{ V_{mf}\frac{[DHAP]}{K_{DHAP}} - V_{mr}\frac{[ | + | <center><math> v = \frac{ V_{mf}\frac{[DHAP]}{K_{DHAP}} - V_{mr}\frac{[Gly3P]}{K_{Gly3P}} }{1 + \frac{[DHAP]}{K_{DHAP}} + \frac{[Gly3P]}{K_{Gly3P}} } </math></center> |
Revision as of 18:22, 26 February 2014
This enzyme rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis.
Chemical equation
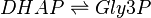
Rate equation
Reversible Michaelis-Menten is used
![v = \frac{ V_{mf}\frac{[DHAP]}{K_{DHAP}} - V_{mr}\frac{[Gly3P]}{K_{Gly3P}} }{1 + \frac{[DHAP]}{K_{DHAP}} + \frac{[Gly3P]}{K_{Gly3P}} }](/wiki/images/math/c/4/3/c43ea2245f63ddca8689602949bdf9f4.png)